Strategies to overcome tamoxifen resistance in breast cancer
Farzana Y Zaman
Saturday, June 4th, 2016
The selective oestrogen receptor modulator (SERM), tamoxifen, is pivotal in treating oestrogen receptor positive (ER+) breast cancers—the most common subtype of breast cancer. As the first targeted therapy for breast cancer, tamoxifen remains the gold standard of adjuvant endocrine therapy. However, this important drug has its limitations: its efficacy is frequently hampered by the phenomenon of tamoxifen resistance.
This article provides an overview of ER+ breast cancer biology relevant to understanding the complexities of tamoxifen resistance. The principal aim is to review the current literature on the mechanisms underpinning tamoxifen resistance and emerging strategies to overcome this challenge, with a focus on those with the greatest translational potential.
Numerous molecular mechanisms of tamoxifen resistance have been proposed and investigated. Well-studied, clinically relevant mechanisms include growth factor receptor signalling, kinase pathway aberrations, cell-cycle dysregulation and epigenetic involvement. Other areas of relevance include the development of new generation SERMs and preclinical studies with novel agents. There is also increasing research into the roles of ER coregulator proteins and cancer stem cells.
Altogether, these areas of interest represent promising opportunities in overcoming the challenge of tamoxifen resistance and ameliorating current breast cancer therapies.
Introduction
Breast cancer is one of the most commonly diagnosed cancers in Australian women. [1] Importantly, breast cancer is a heterogenous disease. It exhibits a wide spectrum of clinical, histopathological and molecular features, which impact prognosis, survival, and response to treatment. In 1896, Sir George Beatson made a landmark discovery: bilateral oophorectomy resulted in tumour remission in a significant proportion of women with metastatic breast cancer. [2] This finding gave birth to the theory that the ovarian hormone, oestrogen, is involved in stimulating the growth of some breast cancers. Indeed, on a molecular level, 70-75% of invasive breast cancers are oestrogen receptor positive (ER+) and proliferate in response to oestrogen, [3] as Beatson deduced.
Patients with ER+ tumours tend to have good prognoses and long-term survival, with a 5-year survival rate of 80-85% in the curative setting. In comparison, ER negative (ER-) cancers are associated with earlier relapses and a lower 5-year survival rate. [3] These discrepancies are partly attributable to availability of effective endocrine therapies, and demonstrate the marked efficacy of such targeted therapies.
Methods
A broad literature review was undertaken on Ovid MEDLINE and PubMed using combinations of the search terms ‘tamoxifen resistance OR endocrine resistance’; ‘mechanism’; and ‘breast cancer’. Limits were set to include articles written in English published since 2000. The search was then further refined to clinical trials published since 2010.
Background
Endocrine therapy
Beatson’s findings led to the naissance of endocrine therapies: drugs that either inhibit oestrogen synthesis or block the oestrogen receptor (ER). Indeed, these therapies have revolutionised breast cancer management. There are three main classes of endocrine therapies (Table 1) used as adjuvants to surgery, radiotherapy, and chemotherapy in treating ER+ breast cancers.
Tamoxifen, in particular, has changed the landscape of breast cancer treatment since its discovery over three decades ago. In 1998, a meta-analysis by the Early Breast Cancer Trialists’ Collaborative Group confirmed that adjuvant tamoxifen treatment substantially improved 10-year survival rates in women with ER+ breast tumours. [4] It has achieved a 39% reduction in disease recurrence and 31% reduction in mortality in early-stage ER+ cancers. [5]
Despite newer drugs, such as aromatase inhibitors (AIs) and selective oestrogen receptor degraders (SERDs), tamoxifen remains the cornerstone of endocrine therapy. Current American Society of Clinical Oncology guidelines outline tamoxifen as first-line adjuvant endocrine therapy in pre-menopausal women and, alongside aromatase inhibitors, in postmenopausal women. [6] Until recently, tamoxifen was also recommended for systemic therapy for metastatic hormone-dependent breast cancers. [7] However, AIs are increasingly being used due to the issue of tamoxifen resistance. [8]
Table 1. Endocrine therapies currently used for ER+ breast cancer.
| Class | Example | Mechanism of action |
| Selective oestrogen receptor modulators (SERMs) | Tamoxifen | Partial ER agonist and antagonist Binds ER, modulates downstream gene transcription and function |
| Aromatase inhibitors (AIs) | Steroidal: Anastrozole
Letrozole Exemestane |
Blocks peripheral conversion of adrenal androgens to oestrogen Only successful in women without ovarian function |
| Selective oestrogen receptor degraders (SERDs) | Fulvestrant | Binds to ER, leads to degradation of receptor |
Tamoxifen resistance
Approximately 30% of patients with ER+ tumours fail to respond to tamoxifen from the initiation of therapy, [9,10] which is termed de novo or intrinsic resistance. [11] Another 30-40% of patients receiving adjuvant tamoxifen eventually develop disease progression or recurrence within three to five years; this is known as acquired resistance. [10,12]
Understanding tamoxifen resistance is a major focus of current breast cancer research. Before undertaking a literature review on current strategies to overcome tamoxifen resistance, it is necessary to touch on basic ER biology in breast cancer.
Oestrogen receptor biology
There are two ER isoforms, ERα and ERβ, [13] and both are present in normal breast tissue. ERα is clearly associated with breast carcinogenesis and progression, and is the subtype best measured in assays. In contrast, the role of ERβ in breast cancer is still unclear. [13] Use of the term ER hereon refers to ERα, unless otherwise specified.
The classical pathway of ER signalling involves oestrogen binding to the ligand-binding domain of ER. The ligand-activated ER then binds to oestrogen response elements via essential transcription factors that regulate target gene expression. [14] In breast cancer, oestrogen-mediated activation of the ER pathway leads to another chain of events resulting in altered gene transcription, ultimately producing proteins that drive cell division, differentiation, proliferation, and angiogenesis. Subsequently, this leads to tumour growth and progression. [15] Furthermore, coregulator proteins modulate ER transcriptional activities. These proteins either activate or repress transcription of ER-responsive genes and are known as coactivators and corepressors, respectively. [16]
Mechanisms to overcome tamoxifen resistance
Growth factor receptor signalling
Growth factors are involved in regulating the ER signalling pathway. [17] These include membrane receptor tyrosine kinases such as epidermal growth factor receptor (EGFR) and human epidermal receptor (IGFR) (Figure 1). [17,18] If upregulated, these growth factor pathways can provide ER+ breast cancers with stimuli for growth, proliferation, and survival, even when the ER pathway has been inhibited, thus behaving as ER-independent drivers of tumour growth.
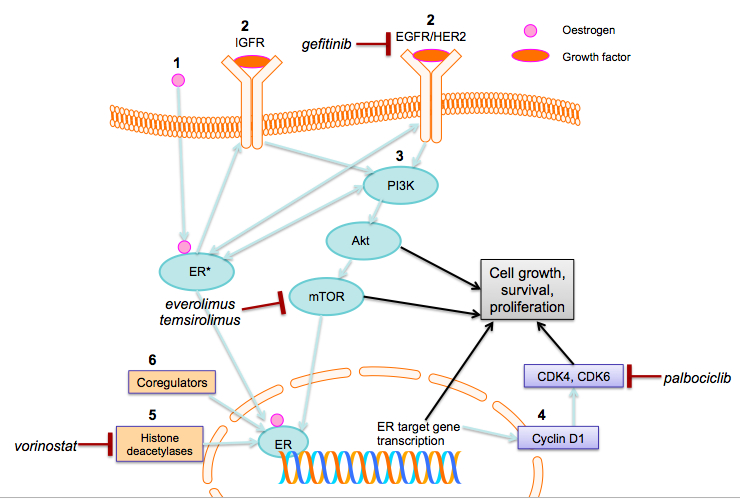
Figure 1: Pathways upregulated in tamoxifen resistance in ER+ breast cancers and associated drug targets. 1. Oestrogen activates the ER. Oestrogen-bound ER activates ER target gene transcription, which is influenced by ER coregulator proteins and enzymes, such as histone deacetylases. 2. Oestrogen-bound ER also activates growth factor receptors (IGFR, EGFR, HER2). 3. This activates key molecules in the PI3K/Akt/mTOR downstream pathway. In turn, this leads to increased cell growth, survival, and proliferation. There is bidirectional crosstalk among the PI3K/Akt/mTOR pathway, EGFR/HER2 and ER. 4. Cyclin D1 is an ER transcriptional target gene that activates CDK4 and CDK6, leading to cell proliferation. 5. Histone deacetylation and 6. ER coregulator proteins modify ER target gene transcription, which also leads to cell growth, survival and proliferation. Gefitinib inhibits EGFR; everolimus and temsirolimus inhibit mTOR activation, palbociclib; palbocilib is a selective CDK4 and CDK6 inhibitor; and vorinostat inhibits histone deacetylation. (Abbreviations: ER, oestrogen receptor; IGFR, insulin-like growth factor receptor; EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; PI3K, phosphatidylinositol-3-kinase; Akt, protein kinase B; mTOR, mammalian target of rapamycin; CDK, cyclin-dependent kinase)
There is a growing body of evidence that increased growth factor signalling contributes to endocrine therapy resistance. The EGFR/HER2 pathway has been strongly implicated. In fact, preclinical and clinical studies demonstrate that tumours overexpressing EGFR or HER2 are less likely to benefit from endocrine therapy. [19] HER2+/ER+ tumours have poorer prognoses, compared to HER2-/ER+ tumours. [3] In xenograft models, HER2 overexpression leads to tamoxifen-stimulated growth, a potential mechanism conferring intrinsic resistance. Direct interactions between ER and HER2 protect HER2+ cancer cells from tamoxifen-induced apoptosis. [17,20] Consequently, blocking this pathway with the HER2 antibody, trastuzumab, restores tamoxifen sensitivity in resistant cells. [21]
The EGFR/HER2 pathway is also involved in acquired tamoxifen resistance. In vitro, long-term tamoxifen treatment leads to stronger EGFR and HER2 expression at the time of drug resistance. A marked increase in EGFR expression is seen in xenograft ER+ tumours with acquired resistance. [12] It has also been shown that EGFR downstream elements that stimulate proliferation and cell survival are overactive in tamoxifen resistant cells. Gefitinib is a selective inhibitor of the tyrosine kinase domain of the EGFR (Figure 1). Adding gefitinib to tamoxifen has been shown to significantly delay the onset of resistance, in vitro. [22]
Based on these promising findings, several phase II clinical trials have combined endocrine and targeted inhibitor therapies. Recent preliminary studies confirm that adding gefitinib to tamoxifen is beneficial compared to placebo, and the combination warrants further clinical investigation. [23]
Kinase pathways
The phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt) /mammalian target of rapamycin (mTOR) pathway is an important downstream target in ER+ breast cancer (Figure 1). It has a key role in breast carcinogenesis, and has been linked to endocrine therapy resistance. The mTOR protein controls cellular processes such as growth, survival, and proliferation. Activating mutations in PI3K are known to be oncogenic. [24] Indeed, PIK3CA gene mutations are amongst the commonest somatic mutations in breast cancer and are more frequently identified in ER+ breast cancers than ER- cancers. [25,26]
Crosstalk between ER and the PI3K/Akt/mTOR pathway increases oestrogen-induced transcriptional activity, contributing to tamoxifen resistance. [27] In vitro, the PI3K pathway is activated in response to oestrogen depletion, leading to acquired hormone-resistant breast cancer cells. [28] The drug temsirolimus prohibits mTOR activation, thus acting as an mTOR inhibitor (Figure 1). Temsirolimus can restore tamoxifen sensitivity in breast cancer cells. Phase II clinical trials demonstrate the beneficial effects of adding another similarly acting mTOR inhibitor, everolimus, to tamoxifen in patients with metastatic disease that is resistant to AIs. [29] Likewise, clinical trials of everolimus in combination with endocrine therapies (exemestane and letrozole) have led to the first mTOR-inhibitor being approved for postmenopausal women with advanced ER+/HER2- breast cancer. [29,30] Altogether, this points strongly at the role of mTOR inhibitors in overcoming endocrine resistance.
Histone deacetylases
Epigenetic mechanisms, such as histone acetylation and hypoacetylation, modify gene expression. Both histone acetylation and hypoacetylation can contribute to oncogenesis, depending on the target gene. Histone deacetylases (HDACs) are the primary enzymes involved in histone hypoacetylation, and have been associated with breast cancer. Importantly, studies have shown that HDACs interact with the ER signalling pathway (Figure 1), but the precise mechanism remains to be elucidated. [31] Epigenetic modulation of ER signalling by HDAC inhibition is another promising strategy to combat tamoxifen resistance. The drug vorinostat binds to the active site of HDACs, to inhibit their action (Figure 1). In vitro, vorinostat downregulates ER transcription and potentiates apoptotic cell death in ER+ cells. [32] In Phase II clinical studies, the combination of vorinostat and tamoxifen appears well tolerated and provides encouraging results for reversing hormone resistance. [33]
Cyclins and cyclin-dependent kinases
Cyclins and cyclin-dependent kinases (CDKs) are involved in cell-cycle regulation. The cyclin D1 gene is a direct transcriptional target of ER signalling. Cyclin D1 activates cyclin-dependent kinases 4 and 6 (CDK4 and CDK6), thus inactivating the retinoblastoma tumour suppressor protein. In turn, this promotes cell-cycle entry and proliferation. [34] In vitro, endocrine therapy-resistant breast cancer models exhibit an uncoupling of ER signalling and cell cycle progression: cyclin D1 activity is maintained despite effective blockade of ER with tamoxifen. There is evidence suggesting that resistant cancers remain dependent on cyclin D1-CDK4 to drive proliferation. [35] Palbociclib is a selective CDK4 and CDK6 inhibitor (Figure 1) [34] that has been studied as a potential reverser of endocrine resistance. In vitro studies and phase II trials of palbociclib as a synergistic therapy with letrozole in resistant tumours demonstrated that this combination is associated with longer progression-free survival. [36-38] A recent phase III trial involving patients with advanced endocrine therapy-resistant ER+ tumours concluded that palbociclib combined with fulvestrant was more effective than fulvestrant alone. [39] Though these studies do not focus on tamoxifen resistance per se, the results suggest a promising role for this drug in improving treatment outcomes for ER+ cancers.
Alternative endocrine therapies
Aromatase inhibitors (Table 1) are now standard treatment options for postmenopausal women with ER+ tumours. It is commonly accepted that third generation AIs have equivalent or superior efficacy to tamoxifen in postmenopausal women. [8,40-42] Furthermore, AIs appear to be effective in some postmenopausal patients with acquired tamoxifen resistance. [43] However, AI resistance is proving to be as significant a problem as tamoxifen resistance. There is increasing research into the mechanisms behind AI resistance and trials combining targeted therapies with AIs. [44]
The SERD, fulvestrant, binds to the ER, degrades the receptor and inhibits its signalling pathways (Table 1). Unlike tamoxifen, it does not have agonist effects but has comparable efficacy to tamoxifen in postmenopausal women. [45] Importantly, it is not cross-resistant to tamoxifen and is as effective as anastrozole in treating postmenopausal women with acquired tamoxifen resistance. [46, 47] However, the efficacy of fulvestrant is dose-dependent [48] and more studies are needed to optimise treatments. Nevertheless, novel ER antagonists are a growing area in the pursuit to overcome endocrine resistance.
One such novel antagonist is TAS-108, a synthetic ER ligand with pure antagonistic activity that promotes corepressor recruitment without preventing DNA-binding activity. In preclinical studies, TAS-108 successfully inhibits tamoxifen-resistant tumour growth. [49] Phase II trials in postmenopausal patients show that it leads to observable clinical benefits in a proportion of patients. Additionally, it is well tolerated and not associated with significant changes in hormone levels or bone metabolism markers, as is the case with tamoxifen. [50]
New generation SERMs
Another strategy hinges on the development of new SERMs to inhibit ER signalling pathways. Two classes of alternative SERMs have been developed: tamoxifen-like compounds (idoxifene, toremifene, and droloxifine) and fixed-ring compounds (raloxifene, arzoxifene, and EM-800). [51,52]
As a class, new generation SERMs have greater binding affinity for ER and reduced agonist activity, compared to tamoxifen. Preclinical studies (in vitro and in vivo) have demonstrated that some alternative SERMs are more effective than tamoxifen in inhibiting ER+ tumour growth, including tamoxifen-resistant tumours. [53-55] However, this efficacy is yet to be recapitulated in clinical trials. [56] The prospect of clinically useful alternative SERMs is clouded by the fact that most known SERMs display a high level of cross-resistance with tamoxifen. [57] Indubitably, further research is required.
Novel agents in preclinical investigation
VEGF inhibitors
Angiogenesis is a hallmark of tumour growth and invasion. This process is modulated by the vascular endothelial growth factor (VEGF) family of growth factors and their associated receptors. Oestrogen enhances angiogenesis via VEGF release. [58] This oestrogen-dependent production of VEGF can be ablated by tamoxifen in tamoxifen-sensitive breast cancer cells. However, in tamoxifen-resistant cells, the VEGF/vascular endothelial growth factor receptor 2 (VEGFR2) signalling loop remains active, despite anti-oestrogenic treatment. [59]
High VEGF/VEGFR2 expression with concomitant elevated p38 mitogen-activated protein kinase activity is associated with poor outcomes in tamoxifen-treated cancers. Inhibition of p38 increases the inhibitory effect of tamoxifen in tamoxifen-resistant cells. [59] In murine xenograft models of tamoxifen-resistant ER+ tumours, low doses of the small-molecule VEGFR2 antagonist, brivanib alaninate, combined with tamoxifen, retards tumour growth and maximises therapeutic efficacy. [60] It remains to be seen whether this approach is effective in the clinic.
Src inhibitors
Src is a membrane-associated non-receptor tyrosine kinase belonging to the Src family kinase group (SFK). [61] Through their involvement in regulating signals from transmembrane receptor-associated tyrosine kinases and in activating intracellular target proteins, [62] SFKs modulate cell survival, proliferation, differentiation and angiogenesis. [63] Src also coordinates ER signalling and plays a role in its non-genomic effects. [63] Studies demonstrate associations between endocrine resistance, elevated Src activity and more aggressive tumour phenotypes. [64,65] Blocking interactions between ER and Src inhibits downstream cellular pathways, leading to decreased cell growth. [66] In vitro, Src inhibitors, which target the peptide substrate site of Src, partially restore response to tamoxifen in resistant cells [67] and reduce their invasive ability. [65] The combination of Src and EGFR inhibition has been shown to result in further growth inhibition in tamoxifen-resistant breast cancer cells. [65] These preclinical results suggest a promising role for Src inhibitors in overcoming tamoxifen resistance.
Notch inhibitors
Notch receptors belong to a signalling pathway involved in cell-to-cell communication and regulation of differentiation, proliferation, and apoptosis. [68] Elevated Notch-1 is associated with poor prognosis in breast cancer. In cell models of ER+ breast cancer, small interfering RNA-mediated Notch inhibition potentiates the effects of tamoxifen. When added to tamoxifen in murine xenograft models, such Notch inhibition leads to regression of ER+ tumours. [69]
Other areas of interest
Oestrogen receptor transcription factors and coregulator proteins
ER activity is influenced by ER transcription factors and coregulatory proteins. The ER pioneer transcription factor, forkhead box protein A1 (FOXA1), and transcription factor GATA3 regulate binding between ER and chromatin, which is vital for tamoxifen’s activity on ER. [70] Indeed, FOXA1 has been identified as a positive prognostic marker of ER+ breast cancer and an indicator of endocrine therapy response. [71] GATA3 has received attention, as it is one of three genes mutated in over 10% of breast cancers, however, it is yet to be clearly linked with endocrine response. [72]
ER coregulators have long been known to play a part in tamoxifen’s function. When tamoxifen binds to ER in the breast, the resulting receptor conformation favours corepressor recruitment, consequently blocking the proliferative actions of ER signalling. In contrast, oestrogen-bound ER favours coactivator recruitment. [73]
Increased coactivator and decreased corepressor expression is frequently seen in breast tumourigenesis. [74] Coactivator gene AIB1 is amplified in 50% of primary breast tumours and correlates with ER-positivity. [75] AIB1 overexpression is also associated with poorer outcomes in patients receiving tamoxifen, pointing at a link to tamoxifen-resistant tumours. [76] Experimental overexpression of the coactivator, nuclear receptor coactivator 1 (NCOA1), increases tamoxifen’s agonist activity. [77] This bolsters the belief that coactivator overexpression contributes to endocrine resistance by enhancing the unfavourable ER agonist activity of tamoxifen.
Conversely, downregulation of the corepressor, nuclear receptor corepressor (NCOR), is observed in xenograft models with acquired tamoxifen resistance. [78] An imbalance in coactivator and corepressor gene expression may impair tamoxifen activity by eliminating its antagonistic effect. [74] Indeed, coregulators appear to be an exciting future drug target.
Cancer stem cells
The cancer stem cell (CSC) hypothesis has established another area of investigation. Tumour-initiating CSCs drive cancer progression and metastasis and may be partly responsible for resistance. In the normal breast, stem cells possess an ER- phenotype. It is postulated that a remaining pool of ER- CSCs in tumour areas continue to develop over growth-arrested ER+ cells. In essence, this converts the bulk of tumour cells from ER+ to ER-. [79] Most current therapies fail to eliminate CSCs, thus potentiating such growth.
Tamoxifen-resistant cells have a larger CSC population than sensitive cells. [80] Furthermore, several groups have identified dysregulated stem cell signalling mechanisms and overexpressed stem cell markers in tamoxifen-resistant cells and tumours that have failed endocrine therapy. [80,81] Significantly, in preclinical studies, downregulation or inhibition of these pathways sensitises resistant cells or decreases progenitor populations. [80,81] Therefore, CSCs hold great potential as putative targets for treating tamoxifen-resistant breast cancers.
Future
It is a fundamental tenet of oncology that no single drug will “cure” cancer. Likewise, no single drug will overcome tamoxifen resistance. It is resoundingly clear that there is no single mechanism underlying tamoxifen resistance. Rather, it involves complex molecular interactions and crosstalk between pathways. The key to overcoming resistance lies in the development of combination therapies. Many promising clinical trials in this area centre on combining tamoxifen with drugs targeting postulated molecular pathways that modulate ER effects. Other approaches aim to build on the principles of tamoxifen therapy by optimising its pharmacology and elucidating methods to increase its favourable antagonist actions.
Moreover, multiple mechanisms may contribute to resistance in an individual patient. This ties in with another principle of modern oncology: the importance of personalised medicine. The concomitant challenge lies in identifying prognostic biomarkers of intrinsic resistance before commencing therapy, and those of acquired resistance as early as possible. The advent of next generation sequencing has already enabled the identification of some genetic and molecular signatures. Hand-in-hand with this, comes the potential need for rebiopsy to detect changes in tumour biology following directed therapies, an area which requires careful consideration. Undoubtedly, in the future, clinically useful biomarkers will facilitate personalised, targeted therapies to overcome the issue of tamoxifen resistance.
Conclusion
There are numerous approaches to addressing tamoxifen resistance. Altogether, this plethora of information sheds light on the clinical conundrum. More importantly, it draws us closer to a multi-faceted strategy; the clinical trials highlighted above are testament to this. With greater research and collaboration, areas currently in the basic scientific or preclinical pipeline may be translated to clinical trials. This will provide clinicians and patients further hope in combating the phenomenon of tamoxifen resistance with more effective therapeutic options.
Conflicts of Interest
None declared.
References
[1] Australian Institute of Health and Welfare. Cancer in Australia: an overview 2014, Cancer series no. 78. Canberra: AIHW; 2014. Cat. no. CAN 75.
[2] Beatson G. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment, with illustrative cases. Lancet. 1896; 148(3803):162-5.
[3] Sandhu R, Parker JS, Jones WD, Livasy CA, Coleman WB. Microarray-based gene expression profiling for molecular classification of breast cancer and identification of new targets for therapy. Lab Medicine. 2010;41(6):364-72.
[4] Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351(9114):1451-67.
[5] Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687-717.
[6] Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update. J Clin Oncol. 2014;32(21):2255-69.
[7] Bernard-Marty C, Cardoso F, Piccart MJ. Facts and controversies in systemic treatment of metastatic breast cancer. Oncologist. 2004;9(6):617-32.
[8] Paridaens R, Dirix L, Lohrisch C, Beex L, Nooij M, Cameron D, et al. Mature results of a randomized phase II multicenter study of exemestane versus tamoxifen as first-line hormone therapy for postmenopausal women with metastatic breast cancer. Ann Oncol. 2003;14(9):1391-8.
[9] Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr Relat Cancer 2004;11(4):643-58.
[10] Haldosén L-A, Zhao C, Dahlman-Wright K. Estrogen receptor beta in breast cancer. Mol Cell Endocrinol. 2014;382(1):665-72.
[11] Riggins RB, Schrecengost RS, Guerrero MS, Bouton AH. Pathways to tamoxifen resistance. Cancer Lett. 2007;256(1):1-24.
[12] Massarweh S, Schiff R. Resistance to endocrine therapy in breast cancer: exploiting estrogen receptor/growth factor signaling crosstalk. Endocr Relat Cancer 2006;13(Supplement 1):S15-S24.
[13] Speirs V, Skliris GP, Burdall SE, Carder PJ. Distinct expression patterns of ERα and ERβ in normal human mammary gland. J Clin Pathol. 2002;55(5):371-4.
[14] Williams C, Lin CY. Oestrogen receptors in breast cancer: basic mechanisms and clinical implications. Ecancermedicalscience. 2013;7:370.
[15] Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002;346(5):340-52.
[16] Osborne CK, Schiff R, Fuqua SA, Shou J. Estrogen receptor: current understanding of its activation and modulation. Clin Cancer Res. 2001;7(12 Suppl):4338s-42s.
[17] Shou J, Massarweh S, Osborne C, Wakeling A, Ali S, Weiss H, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926 – 35.
[18] Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233-47.
[19] Arpino G, Green SJ, Allred DC, Lew D, Martino S, Osborne CK, et al. HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: a Southwest Oncology Group study. Clin Cancer Res. 2004;10(17):5670-6.
[20] Chung Y-L, Sheu M-L, Yang S-C, Lin C-H, Yen S-H. Resistance to tamoxifen-induced apoptosis is associated with direct interaction between Her2/neu and cell membrane estrogen receptor in breast cancer. Int J Cancer. 2002;97(3):306-12.
[21] Chen B, Wang Y, Kane SE, Chen S. Improvement of sensitivity to tamoxifen in ER-positive and Herceptin-resistant breast cancer cells. J Mol Endocrinol. 2008;41(5):367-77.
[22] Gee JM, Harper ME, Hutcheson IR, Madden TA, Barrow D, Knowlden JM, et al. The antiepidermal growth factor receptor agent gefitinib (ZD1839/Iressa) improves antihormone response and prevents development of resistance in breast cancer in vitro. Endocrinology. 2003;144(11):5105-17.
[23] Osborne CK, Neven P, Dirix LY, Mackey JR, Robert J, Underhill C, et al. Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II study. Clin Cancer Res. 2011;17(5):1147-59.
[24] Vinayak S, Carlson RW. mTOR inhibitors in the treatment of breast cancer. Oncology. 2013;27(1):38-44, 6, 8 passim.
[25] Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4(12):988-1004.
[26] Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo W-L, Davies M, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68(15):6084-91.
[27] Tokunaga E, Hisamatsu Y, Tanaka K, Yamashita N, Saeki H, Oki E, et al. Molecular mechanisms regulating the hormone sensitivity of breast cancer. Cancer Sci. 2014;105(11):1377-83.
[28] Beelen K, Opdam M, Severson TM, Koornstra RH, Vincent AD, Wesseling J, et al. Phosphorylated p-70S6K predicts tamoxifen resistance in postmenopausal breast cancer patients randomized between adjuvant tamoxifen versus no systemic treatment. Breast Cancer Res. 2014;16(1):R6.
[29] Bachelot T, Bourgier C, Cropet C, Ray-Coquard I, Ferrero J-M, Freyer G, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J. Clin. Oncol.. 201; 30(22):2718-24.
[30] Baselga J, Campone M, Piccart M, Burris HA, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2012;366(6):520-9.
[31] Citro S, Miccolo C, Meloni L, Chiocca S. PI3K/mTOR mediate mitogen-dependent HDAC1 phosphorylation in breast cancer: a novel regulation of estrogen receptor expression. J Mol Cell Biol. 2015;7(2):132-42.
[32] Hodges-Gallagher L, Valentine CD, Bader SE, Kushner PJ. Inhibition of histone deacetylase enhances the anti-proliferative action of antiestrogens on breast cancer cells and blocks tamoxifen-induced proliferation of uterine cells. Breast Cancer Res Treat. 2007;105(3):297-309.
[33] Munster PN, Thurn KT, Thomas S, Raha P, Lacevic M, Miller A, et al. A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br J Cancer. 2011;104(12):1828-35.
[34] Rocca A, Farolfi A, Bravaccini S, Schirone A, Amadori D. Palbociclib (PD 0332991): targeting the cell cycle machinery in breast cancer. Exp Opin Pharmacother. 2014;15(3):407-20.
[35] Thangavel C, Dean JL, Ertel A, Knudsen KE, Aldaz CM, Witkiewicz AK, et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr-Relat Cancer. 2011;18(3):333-45.
[36] DeMichele A, Clark AS, Tan KS, Heitjan DF, Gramlich K, Gallagher M, et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res. 2015;21(5):995-1001.
[37] Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77-R.
[38] Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. The Lancet Oncol. 2015;16(1):25-35.
[39] Turner NC, Ro J, André F, Loi S, Verma S, Iwata H, et al. Palbociclib in Hormone-Receptor–Positive Advanced Breast Cancer. N Engl J Med 2015; 373(3)209-19.
[40] Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer JU, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005;23(22):5108-16.
[41] Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21(11):2101-9.
[42] Ellis MJ, Coop A, Singh B, Mauriac L, Llombert-Cussac A, Janicke F, et al. Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol. 2001;19(18):3808-16.
[43] Buzdar A, Douma J, Davidson N, Elledge R, Morgan M, Smith R, et al. Phase III, multicenter, double-blind, randomized study of letrozole, an aromatase inhibitor, for advanced breast cancer versus megestrol acetate. J Clin Oncol. 2001;19(14):3357-66.
[44] Ma CX, Reinert T, Chmielewska I, Ellis MJ. Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer. 2015;15(5):261-75.
[45] Howell A, Robertson JFR, Abram P, Lichinitser MR, Elledge R, Bajetta E, et al. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol. 2004;22(9):1605-13.
[46] Chia S, Gradishar W. Fulvestrant: expanding the endocrine treatment options for patients with hormone receptor-positive advanced breast cancer. Breast. 2008;17 Suppl 3:S16-21.
[47] Robertson JF, Llombart-Cussac A, Rolski J, Feltl D, Dewar J, Macpherson E, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol. 2009; 27(27):4530-5.
[48] Young OE, Renshaw L, Macaskill EJ, White S, Faratian D, Thomas JS, et al. Effects of fulvestrant 750mg in premenopausal women with oestrogen-receptor-positive primary breast cancer. Eur J Cancer. 2008; 44(3):391-9.
[49] Buzdar AU. TAS-108: a novel steroidal antiestrogen. Clin Cancer Res. 2005;11(2):906s-8s.
[50] Saeki T, Noguchi S, Aogi K, Inaji H, Tabei T, Ikeda T. Evaluation of the safety and tolerability of oral TAS-108 in postmenopausal patients with metastatic breast cancer. Ann Oncol . 2009;20(5):868-73.
[51] Lewis-Wambi JS, Jordan VC. Treatment of postmenopausal breast cancer with Selective Estrogen Receptor Modulators (SERMs). Breast Dis. 2005;24:93-105.
[52] Jiang Q, Zheng S, Wang G. Development of new estrogen receptor-targeting therapeutic agents for tamoxifen-resistant breast cancer. Future Med Chem. 2013; 5(9):1023-35.
[53] Greenberger LM, Annable T, Collins KI, Komm BS, Lyttle CR, Miller CP, et al. A new antiestrogen, 2-(4-hydroxy-phenyl)-3-methyl-1-[4-(2-piperidin-1-yl-ethoxy)-benzyl]-1H-indol-5-o l hydrochloride (ERA-923), inhibits the growth of tamoxifen-sensitive and -resistant tumors and is devoid of uterotropic effects in mice and rats. J Clin Oncol. 2001;7(10):3166-77.
[54] Freddie CT, Larsen SS, Bartholomaeussen M, Lykkesfeldt AE. The effect of the new SERM arzoxifene on growth and gene expression in MCF-7 breast cancer cells. Mol Cell Endocrinol. 2004;219(1-2):27-36.
[55] Johnston SR, Gumbrell LA, Evans TR, Coleman RE, Smith IE, Twelves CJ, et al. A cancer research (UK) randomized phase II study of idoxifene in patients with locally advanced/metastatic breast cancer resistant to tamoxifen. Cancer Chemother Pharmacol. 2004;53(4):341-8.
[56] Dowsett M, Dixon JM, Horgan K, Salter J, Hills M, Harvey E. Antiproliferative effects of idoxifene in a placebo-controlled trial in primary human breast cancer. J Clin Oncol. 2000;6(6):2260-7.
[57] Gao ZO, Gao ZP, Fields JZ, Boman BM. Development of cross-resistance to tamoxifen in raloxifene-treated breast carcinoma cells. Anticancer Res. 2002;22(3):1379-83.
[58] Hyder SM. Sex-steroid regulation of vascular endothelial growth factor in breast cancer. Endocr-Relat. Cancer. 2006;13(3):667-87.
[59] Aesoy R, Sanchez BC, Norum JH, Lewensohn R, Viktorsson K, Linderholm B. An autocrine VEGF/VEGFR2 and p38 signaling loop confers resistance to 4-hydroxytamoxifen in MCF-7 breast cancer cells. Mol Cancer Res. 2008; 6(10):1630-8.
[60] Patel RR, Sengupta S, Kim HR, Klein-Szanto AJ, Pyle JR, Zhu F, et al. Experimental treatment of estrogen receptor (ER) positive breast cancer with tamoxifen and brivanib alaninate, a VEGFR-2/FGFR-1 kinase inhibitor: a potential clinical application of angiogenesis inhibitors. Eur. J. Cancer. 2010;46(9):1537-53.
[61] Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23(48):7906-9.
[62] Belsches-Jablonski AP, Biscardi JS, Peavy DR, Tice DA, Romney DA, Parsons SJ. Src family kinases and HER2 interactions in human breast cancer cell growth and survival. Oncogene. 2001;20(12):1465-75.
[63] Mayer EL, Krop IE. Advances in targeting Src in the treatment of breast cancer and other solid malignancies. Clin Cancer Res. 2010; 16(14):3526-32.
[64] Riggins RB, Thomas KS, Ta HQ, Wen J, Davis RJ, Schuh NR, et al. Physical and functional interactions between Cas and c-Src induce tamoxifen resistance of breast cancer cells through pathways involving epidermal growth factor receptor and signal transducer and activator of transcription 5b. Cancer Res. 2006;66(14):7007-15.
[65] Hiscox S, Morgan L, Green TP, Barrow D, Gee J, Nicholson RI. Elevated Src activity promotes cellular invasion and motility in tamoxifen resistant breast cancer cells. Breast Cancer Res Treat. 2006;97(3):263-74.
[66] Varricchio L, Migliaccio A, Castoria G, Yamaguchi H, de Falco A, Di Domenico M, et al. Inhibition of estradiol receptor/Src association and cell growth by an estradiol receptor α tyrosine-phosphorylated peptide. Mol Cancer Res. 2007; 5(11):1213-21.
[67] Anbalagan M, Carrier L, Glodowski S, Hangauer D, Shan B, Rowan BG. KX-01, a novel Src kinase inhibitor directed toward the peptide substrate site, synergizes with tamoxifen in estrogen receptor alpha positive breast cancer. Breast Cancer Res Treat. 2012;132(2):391-409.
[68] Miele L, Golde T, Osborne B. Notch signaling in cancer. Curr Mol Med. 2006; 6(8):905-18.
[69] Rizzo P, Miao H, D’Souza G, Osipo C, Song LL, Yun J, et al. Cross-talk between notch and the estrogen receptor in breast cancer suggests novel therapeutic approaches. Cancer Res. 2008;68(13):5226-35.
[70] Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS. FOXA1 is a critical determinant of estrogen receptor function and endocrine response. Nat Genet. 2011; 43(1):27-33.
[71] Mehta R, Jain R, Leung S, Choo J, Nielsen T, Huntsman D, et al. FOXA1 is an independent prognostic marker for ER-positive breast cancer. Breast Cancer Res Treat. 2012;131(3):881-90.
[72] Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61-70.
[73] Heldring N, Nilsson M, Buehrer B, Treuter E, Gustafsson J-Å. Identification of tamoxifen-induced coregulator interaction surfaces within the ligand-binding domain of estrogen receptors. Mol Cell Biol. 2004;24(8):3445-59.
[74] Girault I, Bieche I, Lidereau R. Role of estrogen receptor alpha transcriptional coregulators in tamoxifen resistance in breast cancer. Maturitas. 2006;54(4):342-51.
[75] Bautista S, Vallès H, Walker RL, Anzick S, Zeillinger R, Meltzer P, et al. In breast cancer, amplification of the steroid receptor coactivator gene AIB1 is correlated with estrogen and progesterone receptor positivity. Clin Cancer Res. 1998; 4(12):2925-9.
[76] Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SAW, et al. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003; 95(5):353-61.
[77] Smith CL, Nawaz Z, O’Malley BW. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-Hydroxytamoxifen. Mol Endocrinol. 1997;11(6):657-66.
[78] Lavinsky RM, Jepsen K, Heinzel T, Torchia J, Mullen T-M, Schiff R, et al. Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc Natl Acad Sci U.S.A. 1998;95(6):2920-5.
[79] O’Brien C, Howell S, Farnie G, Clarke R. Resistance to endocrine therapy: are breast cancer stem cells the culprits? J Mammary Gland Biol Neoplasia. 2009; 14(1):45-54.
[80] Dubrovska A, Hartung A, Bouchez LC, Walker JR, Reddy VA, Cho CY, et al. CXCR4 activation maintains a stem cell population in tamoxifen-resistant breast cancer cells through AhR signalling. Br J Cancer. 2012;107(1):43-52.
[81] Piva M, Domenici G, Iriondo O, Rabano M, Simoes BM, Comaills V, et al. Sox2 promotes tamoxifen resistance in breast cancer cells. EMBO molecular medicine. 2014;6(1):66-79.