Insights into the mechanism of ‘chemobrain’: deriving a multi-factorial model of pathogenesis
Kok-Ho Ho
Tuesday, September 1st, 2015
Chemotherapy-related cognitive impairment, commonly called ‘chemobrain’, is a potentially debilitating condition that is slowly being recognised. It encompasses a wide range of cognitive domains and can persist up to years after the cessation of chemotherapy. What initially appears to be a straightforward example of neurotoxicity may be a complex interplay between individual susceptibilities and treatment characteristics, the effects of which are perpetuated through mechanisms such as oxidative stress and telomere shortening via cytokines. This article will attempt to propose a multi-factorial model of pathogenesis which may clarify the relationship between these factors and ultimately improve the life of cancer patients through informed decisions during the chemotherapy process.

Introduction
Chemotherapy is a mainstay in modern oncological treatment. Chemotherapeutic drugs are often cytotoxic and this allows cancer cells to be destroyed effectively. However, the systemic nature of chemotherapy means that normal cells are damaged too. If cells in the central nervous system are affected, neurological effects manifesting into cognitive deficits may be evident. The link between chemotherapy and cognitive impairment was first reported by Silberfarb and colleagues in the 1980s. [1] In the past 10-20 years, research in this area further developed due to fairly high rates of cognitive decline in cancer patients receiving chemotherapy. The cognitive sequelae arising from chemotherapy is commonly referred to as ‘chemobrain’.
It is estimated that up to 70-75% of cancer patients have cognitive deficits during and post-chemotherapy, and up to half of these patients will have impairment lasting months or years after treatment. [2,3] Transient cognitive impairment during chemotherapy is usually tolerated but persistence of these symptoms can cause significant psychological stress and affect activities of daily living such as work, education, and social interaction.
Understanding chemotherapy-related cognitive impairment can help guide the choice and dosage/duration of chemotherapeutic drugs and ultimately enable us to improve the quality of life of cancer patients undergoing treatment. This article will briefly examine what is known about ‘chemobrain’ and attempt to propose a multi-factorial model of pathogenesis.
What is ‘chemobrain’?
The cognitive domains involved in ‘chemobrain’ are not fully defined but they are thought to be related to structural and functional changes in the frontal lobes and hippocampus of the brain. [4] Domains affected often include executive functioning, possessing speed, attention/ concentration, as well as verbal and visuospatial memory. [5] While the degree of cognitive decline can be subtle in high-functioning individuals with a resultant cognition within the normal range, even a small decline in cognitive function can significantly reduce the quality of life (QOL) of a cancer patient. This is particularly true for those who experience persistent cognitive deficits. ‘Chemobrain’ can refer to cognitive dysfunction within any time period but recent studies assess cognitive dysfunction in the long-term (i.e. months or years) as immediate cognitive changes are often transient and resolve spontaneously. [6]
Cognitive outcomes in patients undergoing chemotherapy appear to be affected by treatment characteristics. Van Dam and colleagues compared the cognitive function in women receiving high-dose versus standard-dose adjuvant chemotherapy for high-risk breast cancer. The results indicated a dose-related effect whereby a higher proportion of breast cancer patients receiving high-dose chemotherapy had cognitive impairment as compared to patients receiving standard-dose chemotherapy (32% versus 17%). [7] A more recent study by the same team also showed a greater degree of cognitive impairment in breast cancer patients receiving high-dose chemotherapy. [8] However, other studies such as Mehnert et al. and Scherwath et al. did not find any significant difference in post-chemotherapy cognitive function between high-dose and standard-dose groups. [9,10] These inconsistencies are probably due to methodological differences, such as the choice of chemotherapeutic agent and the time of cognitive testing.
The duration and type of regimen were also implicated as possible treatment factors. In early breast cancer patients, the duration of chemotherapy was positively correlated with the degree of cognitive decline. [11] The previously commonplace cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) regime was also shown to increase the incidence of cognitive dysfunction when compared to published test norms of healthy people. [11] In particular, methotrexate is a known neurotoxic agent which affects cell proliferation and blood vessel density in the hippocampus. [12,13] However, similar regimens substituting methotrexate with etoposide or adriamycin also seem to cause cognitive impairment. [14] This brings into question whether a single or combination of chemotherapeutic agents are largely responsible for the cognitive effects.
Are some individuals more susceptible to ‘chemobrain’?
Individual cognitive characteristics
Since ‘chemobrain’ only occurs in a subset of cancer patients, many researchers have postulated that some individuals may be more susceptible than others. Cognitive decline prior to treatment can contribute indirectly to ‘chemobrain’ by establishing a lower baseline cognitive function. Individual characteristics such as poor education, reduced cognitive stimulation, old age, and stress are possible risk factors for developing ‘chemobrain’. Ahles et al. and Adams-Price et al. showed that older patients with low cognitive reserve have a lower processing speed as compared to younger patients. [15,16] This is not unexpected as processing speed decreases with age and cognitive disorders in older patients are generally under-diagnosed.
For example, in the United Sates, about 20% of elderly cancer patients screen positively for cognitive disorders, and dementia is clinically diagnosed in one in two cancer patients above the age of 80. [17,18] Earlier studies that have not shown an association between age and cognitive decline often include younger and more highly-educated individuals, and this could have affected the statistical significance of the results. [19]
Most studies failed to find an association between psychological stress and cognitive dysfunction. This is because many neuropsychological tools measure objective (i.e. cognitive function) rather than subjective cognitive impairment (i.e. cognitive symptoms). The latter is, however, equally important and Jenkins et al. showed that psychological distress can cause subjective cognitive impairment with a consequent significant reduction in QOL. [20] It is difficult to attribute specific proportions of cognitive decline to chemotherapy or emotional distress, but any declines due to stress/grief are likely to be secondary to chemotherapy.
Genetic susceptibility
The apoliprotein E (APOE) and catechol-o-methyltransferase (COMT) genes are involved in neural repair and neurotransmission. [21,22] The human E4 allele of APOE is associated with cognitive disorders such as Alzheimer’s disease, as well as poor prognosis in brain injury and stroke patients. [23,24] One study found that cancer patients with the E4 allele also tend to have poor executive functioning and visuospatial memory irrespective of chemotherapy status. [21]
Interestingly, the brain-derived neurotrophic factor (BDNF) is also implicated as a possible genetic susceptibility factor. The BDNF is involved in neural repair and is preferentially expressed in the frontal lobe and hippocampus. [2] A valine (Val)-to-methionine (Met) amino acid substitution at codon 66 of the BDNF gene confers similar cognitive deficits as those found in APOE E4 carriers. [2,25]
Cognitive performance is dependent on efficient neurotransmission. COMT is required for the metabolism of catecholamines, and this function is especially important in brain regions with low expression of presynaptic dopamine transporter such as the prefrontal cortex. [26] Reduced dopamine level in the prefrontal cortex is associated with a significant decline in executive functioning. COMT-Val allele carriers are rapid metabolisers of dopamine (four times that of COMT-Met allele) and predictably, individuals in the general population with this allele variation were shown to perform poorly in cognitive assessments. [27]
It is worth thinking that chemotherapy may exacerbate cognitive changes in individuals with these specific variations in APOE, BDNF, or COMT.
The current evidence for hormones and cytokines
The fact that cognitive impairment has been shown in diverse types of cancer (breast, CNS, and lymphoma) and even in the presence of the protective blood-brain barrier (BBB), suggests that direct neurotoxicity of chemotherapeutic agents is only partially responsible for ‘chemobrain’. It is believed that a reduction in hormones such as oestrogen and testosterone is associated with cognitive decline. Studies have shown that post-menopausal women undergoing chemotherapy have a poorer cognitive performance as compared to pre-menopausal women. Moreover, despite conflicting results in some studies, pre-menopausal breast cancer patients receiving tamoxifen and chemotherapy are often more cognitively impaired (especially verbal memory and processing speed) than those receiving chemotherapy alone. [28,29] Similar results were also found in males undergoing androgen deprivation therapy (ADT) for prostate cancer. One study found that almost half of the prostate cancer patients undergoing ADT scored 1.5 standard deviations below the mean in more than 2 NP measurements. [30] These observations suggest that oestrogen and testosterone may have neuro-protective roles (such as antioxidant or telomere length maintenance) which are vital to cognitive function. [2]
Cytokine imbalance may also be involved in cognitive decline. Cytokines are responsible for maintaining normal neuronal and glial cell function. They also regulate levels of neurotransmitters such as dopamine and serotonin which are necessary for cognition. [31] Increased levels of pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and interleukin-6 (IL-6), were found in patients receiving chemotherapy for Hodgkin’s disease and breast cancer respectively. [32,33] In particular, an elevated level of IL-6 was associated with a decline in executive functioning. [34] Longitudinal studies of patients receiving immunotherapies consisting of IL-2 or interferon-alpha also found that these therapies result in cognitive decline across a range of domains such as processing speed, spatial ability, and executive functioning. [35] Paradoxically, an elevated level of IL-8 was found to correlate with memory enhancement in acute myelogenous leukemia and myelodyplastic syndrome patients. [34] It is still unclear which cytokines are involved and how they work. Moreover, most studies up to now have focused on acute rather than long-term cognitive changes in cancer patients. Possible roles for hormones and cytokines in chemotherapy-induced cognitive changes will be elaborated in the ‘multi-factorial model’ section.
Is anaemia related to cognitive function?
In anaemic cancer patients, it is hypothesised that low levels of haemoglobin result in ischaemic damage to the brain. Since many chemotherapeutic agents are cardiotoxic, cerebrovascular changes could also further aggravate the hypoxic condition. [36] Both Vearncombe et al. and Jacobsen et al. showed that decline in haemoglobin (Hb) levels is a significant predictor of multiple cognitive impairments (such as attention and visual memory) in patients receiving chemotherapy. [37,38] However, Iconomou et al. found no association between Hb levels and cognition function, although higher Hb levels were significantly correlated with a better QOL. [39] This conflicting result may be attributed to the use of the Mini-Mental State Examination (MMSE), which is in itself too brief and not a very sensitive measure of subtle cognitive impairment. [3] Conversely, Verancombe et al. used a battery of comprehensive neuropsychological assessments to measure different cognitive domains
Establishing a multi-factorial model of ‘chemobrain’
Despite all the research so far, there is still no consensus on how ‘chemobrain’ develops. It is well recognised that oxidative stress is one of the commonest causes of DNA damage in neuronal cells and a number of cognitive disorders such as Alzheimer’s disease and Parkinson’s Disease are associated with it. [40,41] Chemotherapeutic drugs such as Adriamycin are also known to increase production of reactive oxygen species (ROS) and contribute to reduced anti-oxidant capacity. [42] In addition, chemotherapy has often been associated with telomere shortening in patients with breast cancer and haematological malignancies. [43,44] Telomeres shortening can result in adverse cell outcomes such as senescence and apoptosis, and although most CNS cell types are post-mitotic, some such as glial cells are actively dividing and are vulnerable to this process. [45] Based on these observations, it is conceivable that oxidative DNA damage and telomere shortening could form the basis of a model of CNS dysfunction to explain ‘chemobrain’.
As mentioned previously, a lower baseline cognitive function due to individual cognitive characteristics and genetic predisposition can precipitate cognitive difficulties when certain treatment conditions are prevalent. These conditions are not fully understood but may relate to the use of neurotoxic agents, prolonged high-dosage regimens, or simply any therapeutic situation which causes hormonal and/or cytokine imbalances. Cytokines are likely to play a crucial intermediary role linking the neurotrophic effects of chemotherapy to oxidative DNA damage in the CNS as the BBB will limit the entry of most chemotherapeutic agents. [2] Although some animal studies show that a minute dose of these agents can cause cognitive symptoms, such occurrences are typically rare and drug effects may instead follow a dosage-dependent pattern. [46]
In contrast, cytokines can pass through the BBB and mediate their effects freely. Aluise and colleagues proposed a mechanism of pathogenesis whereby Adriamycin causes the release of peripheral tumour necrosis factor-alpha (TNF-α) via cell injury. These cytokines pass through the BBB and induce glial cells to produce more TNF-α, especially in the hippocampus and frontal cortex. Elevated levels of central TNF- α then damage brain cell mitochondria as well as stimulate production of ROS, which results in oxidative stress and DNA damage. [47]
By extrapolation, other pro-inflammatory cytokines such as IL-6 may play similar roles and different chemotherapeutic agents could induce distinct cytokine profiles with varying CNS effects. It is also worth postulating that the same oxidative stress could have led to telomere shortening and subsequently cell apoptosis/senescence. When this occurs in patients who are post-menopausal or undergoing hormonal therapy, the effects of telomere shortening would predictably be more pronounced. As changes in oestrogen status (such as in the transition from pre-menopause to post-menopause) have been linked to fluctuations in levels of cytokines such as IL-6 and alterations in cortisol rhythm are shown to elevate pro-inflammatory cytokine levels, it is possible that interplay between cytokines and hormones could be significant in the pathogenesis of ‘chemobrain’. [48, 49]
How then, does cognitive impairment translate to a diminished QOL? Quantifying cognitive impairment in terms of QOL is difficult due to its objective (assessed by neuropsychological tools) and subjective components (assessed by self-reporting). In some patients, psychological stress coupled with anaemia (and possibly, other side effects of chemotherapy) could have reduced the subjective component of QOL to such an extent that the effects of cognitive difficulties are amplified. This could explain the apparent paradox whereby a subtle change in cognitive function often results in a significant impact on a patient’s quality of life.
Lastly, how do we reconcile the delayed effects of ‘chemobrain’? The immediate effects of chemotherapy are well-established as a result of acute CNS damage but the persistence of cognitive changes has always remained unclear. A study by Han et al. found that systemic administration of the commonly used chemotherapy agent 5-fluorouracil results in a progressively worsening delayed demyelination of the CNS white matter tracts with consequent cognitive impairment. Although this is unlikely to be the only chemotherapy related mechanism of delayed CNS change, it adds to the existing knowledge of prolonged inflammation and vascular damage to the CNS noted in radiotherapy. [50]
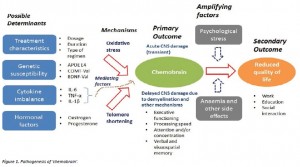
A possible multi-factorial model of ‘chemobrain’ is summarised in Figure 1.
Chemotherapy related cognitive impairment can be affected by a number of possible determinants such as treatment characteristics, genetic susceptibility, cytokine imbalance, and hormonal factors. Mechanisms such as oxidative stress and telomere shortening have been implicated, and studies suggest a mediating role for cytokines. The primary outcome is commonly called ‘chemobrain’, which encompasses a wide range of cognitive domains including executive functioning, processing speed, attention/concentration, as well as verbal and visuospatial memory. The effects of ‘chemobrain’ are both acute and delayed, with the latter thought to involve demyelination of CNS tracts. While ‘chemobrain’ can be subtle, amplifying factors such as psychological stress and anaemia may have a significant impact on the quality of life of a patient in terms of reduced work, education, and social interaction opportunities.
Discussion and conclusion
While good progress has been made in understanding ‘chemobrain’, further research is required in order for clinical interventions to be effective. A multi-prong treatment approach is widely viewed as necessary to manage this condition due to the complexity of the phenomenon. Pharmacological approaches proposed by researchers revolve around reducing oxidative DNA damage and improving neurotransmission. Examples of drugs considered include antioxidants such as zinc sulfate and N-acetyl cysteine, as well as modulators of the catecholaminergic system such as Methylphenidate and Modafinil. [3] Furthermore, cognitive rehabilitation has shown promise in restoring an acceptable baseline level of cognition. [6] However, these interventions are at most speculative and certain mechanistic questions still need to be addressed.
Firstly, it is important to identify further risk factors which could help us identify the cognitive effects of chemotherapy more precisely. This may involve extending our study beyond purely neurological-related genes such as APOE and COMT. Ahles and Saykin have suggested that genes involved in regulating drug transport across the BBB could be involved in ‘chemobrain’. [2] The P-glycoprotein, encoded by the multi-drug resistance 1 (MDR1) gene, is expressed by endothelial cells in the BBB and protects neuronal cells by promoting efflux of drug metabolites. A C3435T polymorphism in exon 26 of the MDR1 gene is associated with reduced efflux capacity of P-glycoprotein and could precipitate buildup of high concentrations of toxic chemotherapy agents. [51] Positron-emission tomography (PET) studies allow monitoring of these concentration changes and may help us understand which drug transporters are involved and how drug doses can affect cognitive function. [52] Evidence of direct chemotherapy neurotoxicity may also be further pinpointed through neuroimaging studies which compare changes in brain integrity on MRI in women treated with chemotherapy compared to cancer patients who did not receive chemotherapy. An example is the study done by Deprez et al., which assessed microstructural changes of cerebral white matter in non-CNS cancer patients. [53]
Secondly, methodological differences between studies pose a serious limitation, which precludes strong conclusions from being derived. Some studies utilize brief assessments, such as the MMSE, which are poor at detecting subtle cognitive changes. There needs to be a battery of NP assessments which are comprehensive yet practical enough to be used in clinical trials (refer to Vardy et al.). [54] In addition, many studies often exclude patients with pre-existing conditions (such as neurological disorders or learning disabilities) for fear of aggravating post-chemotherapy cognitive impairment. [19] This meant that high-risk patients are left out of the analysis and consequently, the actual proportion of patients experiencing ‘chemobrain’ might be underestimated. It is also essential for studies to establish the pre- chemotherapy baseline cognitive level prior to treatment as those, which recruit individuals regardless of cognitive status tend to yield conflicting results. [3] Moreover, studies should endeavour to compare cognitive impairment in the short-term versus the long-term in order to ascertain that cognitive difficulties are persistent and not transient in nature.
The practical implications of understanding ‘chemobrain’ are forseeable. Chemotherapy regimens can be individualized to fit the physical and psychological constitution of the patient. This helps to improve compliance rate and reduce drop-outs due to adverse treatment-related effects. In addition, the existence of ‘chemobrain’ may favour the diversification of treatment modalities instead of focusing on chemotherapy alone. For example, immunotherapy can be trialed as adjuvant to chemotherapy with the aim of reducing the latter’s side effects and potentiating the overall therapeutic gain, such as in the case of indoximod (an IDO inhibitor) and chemotherapy in metastatic breast cancer.
In conclusion, ‘chemobrain’ is a phenomenon which needs to be studied in depth. Current observations favour a framework whereby individuals experience cognitive difficulties due to a combination of inherent vulnerabilities and chemotherapy-related side effects. There is also increasing recognition that cytokines might play a crucial supporting role in pathogenesis. Emphasis should be placed on identifying further chemotherapy-related risk factors, as well as improving the sensitivity of methodological approaches with the aim of improving the design of chemotherapy regimens to provide a better quality of life.
Acknowledgements
None.
Conflict of interest
None declared.
Correspondence
K Ho: koho2292@uni.sydney.edu.au
References
[1] Silberfarb P. Chemotherapy and cognitive defects in cancer patients. Ann Rev Med 1983;34:35-46.
[2] Ahles Ta, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer 2007;7(3):192-201.
[3] Fardell JE, Vardy J, Johnston IN, Winocur G. Chemotherapy and cognitive impairment: treatment options. Clin Pharmacol Ther 2011;90(3):366-76.
[4] Janelsins MC, Kohli S, Mohile SG, Usuki K, Ahles TA, Morrow GR. An update on cancer- and chemotherapy-related cognitive dysfunction: current status. Semin Oncol 2011;38(3):431-8.
[5] Vardy J, Tannock I. Cognitive function after chemotherapy in adults with solid tumours. Crit Rev Oncol Hematol 2007;63:183-202.
[6] Poppelreuter M, Weis J, Bartsch HH. Effects of specific neuropsychological training programs for breast cancer patients after adjuvant chemotherapy. J Psychosoc Oncol 2009;27(2):274-96.
[7] Van Dam FS, Schagen SB, Muller MJ, Boogerd W, vd Wall E, Droogleever Fortuyn ME, et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high dose versus standard-dose chemotherapy. J Natl Cancer Inst 1998;90(3):210-8.
[8] Schagen SB, Muller MJ, Boogerd W, Mellenbergh GJ, van Dam FS. Change in cognitive function after chemotherapy: a prospective longitudinal study in breast cancer patients. J Natl Cancer Inst 2006;98:1742-5.
[9] Mehnert A, Scherwath A, Schirmer L, Schleimer B, Petersen C, Schulz-Kindermann F, et al. The association between neuropsychological impairment, self-perceived cognitive deficits, fatigue and health related quality of life in breast cancer survivors following standard adjuvant versus high-dose chemotherapy. Patient Educ Couns 2007;66(1):108-18.
[10] Scherwath A, Mehnert A, Schleimer B, Schirmer L, Fehlauer F, Kreienberg R, et al. Neuropsychological function in high-risk breast cancer survivors after stem-cell supported high-dose therapy versus standard-dose chemotherapy: evaluation of long-term treatment effects. Ann Oncol 2006;17(3):415-23.
[11] Wieneke MH, Dienst ER. Neuropsychological assessment of cognitive functioning following chemotherapy for breast cancer. Psychooncology 1995;4:61-6.
[12] Seigers R, Timmermans J, van der Horn HJ, de Vries EF, Dierckx RA, Visser L, et al. Methotrexate reduces hippocampal blood vessel density and actives microglia in rats but does not elevate central cytokine release. Behav Brain Res 2010;207(2):265-72.
[13] Seigers R, Schagen SB, Beerling W, Boogerd W, van Tellingen O, van Dam FS, et al. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav BrainRes 2008;186(2):168-75.
[14] Raffa RB, Duong PV, Finney J, Garber DA, Lam LM, Matthew SS, et al. Is ‘chemo-fog’/’chemo-brain’ caused by cancer chemotherapy? J Clin Pharm Ther 2006;31(2):129-38.
[15] Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol 2010;28(29):4434-40.
[16] Adams-Price CE, Morse LW, Cross GW, Williams M, Wells-Parker E. The effects of chemotherapy on useful field of view (UFOV) in younger and older breast cancer patients. Exp Aging Res 2009;35:220-34.
[17] Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007;29(1-2):125-32.
[18] Rodin MB, Mohile SG. A practical approach to geriatric assessment in oncology. J Clin Oncol 2007;25(14):1936-44.
[19] Rodin G. Accumulating evidence for the effect of chemotherapy on cognition. J Clin Oncol 2012;30(29):3568-9.
[20] Jenkins V, Shillings V, Deutsch G, Bloomfield D, Morris R, Allan S, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer 2006;94(6):828-34.
[21] Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology 2003;12(6):612-9.
[22] Small BJ, Rawson KS, Walsh E, Jim HS, Hughes TF, Iser L, et al. Catechol-O-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer 2011;117(7):1369-76.
[23] Laws SM, Clarnette RM, Taddei K, Martins G, Paton A, Hallmayer J, et al. APOE-epilson4 and APOE-491A polymorphisms in individuals with subjective memory loss. Mol Psychiatry 2002;7(7):768-75.
[24] Nathoo N, Chetty R, van Dellen JR, Barnett GH. Genetic vulnerability following traumatic brain injury: the role of apolipoprotein E. Mol Pathol 2003;56(3):132-6.
[25] Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, et al. The brain-derived neutrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci 2004;24(45):10099-102.
[26] Matsumoto M, Weickert CS, Akil M, Lipska BK, Hyde TM, Herman MM, et al. Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience 2003;116(1):127-37.
[27] McAllister TW, Ahles TA, Saykin AJ, Ferguson RJ, McDonald BC, Lewis LD, et al. Cognitive effects of cytotoxic cancer chemotherapy: predisposing risk factors and potential treatments. Curr Psychiatry Rep 2004;6(5):364-71.
[28] Palmer JL, Trotter T, Joy AA, Carlson LE. Cognitive effects of tamoxifen in pre-menopausal women with breast cancer compared to healthy controls. J Cancer Surviv 2008;2(4):275-82.
[29] Zec RF, Trivedi MA. The effects of estrogen replacement therapy on neuropsychological functioning in postmenopausal women with and without dementia: a critical and theoretical review. Neuropsychol Rev 2002;12(2):65-109.
[30] Mohile SG, Lacy M, Rodin M, Bylow K, Dale W, Meager MR, et al. Cognitive effects of androgen deprivation therapy in an older cohort of men with prostate cancer. Crit Rev Oncol Hematol 2010;75(2):152-9.
[31] Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition—the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc 2002;50(12):2041-56.
[32] Pusztai L, Mendoza TR, Reuben JM, Martinez MM, Willey JS, Lara J, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine 2004;25(3):94-102.
[33] Villani F, Busia A, Villani M, Vismara C, Viviani S, Bonfante V. Serum cytokine in response to chemo-radiotherapy for Hodgkin’s disease. Tumori 2008;94(6):803-8.
[34] Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer 2005;104(4):788-93.
[35] Capuron L, Ravaud A, Dantzer R. Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-alpha treatments in cancer patients. Psychosom Med 2001;63(3):376-86.
[36] Theodoulou M, Seidman D. Cardiac effects of adjuvant therapy for early breast cancer. Semin Oncol 2003;30(6):730-9.
[37] Vearncombe KJ, Rolfe M, Wright M, Pachana NA, Andrew B, Beadle G. Predictors of cognitive decline after chemotherapy in breast cancer patients. J Int Neuropsychol Soc 2009;15(6):951-62.
[38] Jacobsen PB, Garland LL, Booth-Jones M, Donovan KA, Thors CL, Winters E, et al. Relationship of hemoglobin levels to fatigue and cognitive functioning among cancer patients receiving chemotherapy. J Pain Symptom Manage 2004;28(1):7-18.
[39] Iconomou G, Koutras A, Karaivazoglou K, Kalliolias GD, Assimakopoulos K, Argyriou AA, et al. Effect of epoetin alpha therapy on cognitive function in anaemic patients with solid tumours undergoing chemotherapy. Eur J Cancer Care 2008;17(6):535-41.
[40] Fishel ML, Vasko MR, Kelley MR. DNA repair in neurons: so if they don’t divide what’s to repair? Mutat Res 2007;614(1-2):24-36.
[41] Mariani E, Polidori MC, Cherubini A, Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular disease: an overview. J Chromatogr B Analyt Technol Biomed Life Sci 2005;827(1):65-75.
[42] Tsang WP, Chau SP, Kong SK, Fung KP, Kwok TT. Reactive oxygen species mediate doxorubicin induced p53-independent apoptosis. Life Sci 2003;73(16):2047-58.
[43] Schroder CP, Wisman GBA, de Jong S, van der Graaf WTA, Ruiters MHJ, Mulder NH, et al. Telomere length in breast cancer patients before and after chemotherapy with or without stem cell transplantation. Br J Cancer 2001;84(10):1348-53.
[44] Lahav M, Uziel O, Kestenbaum M, Fraser A, Shapiro H, Radnay J, et al. Nonmyeloablative conditioning does not prevent telomere shortening after allogeneic stem cell transplantation. Transplantation 2005;80(7):969-76.
[45] Flanary BE, Streit WJ. Progressive telomere shortening occurs in cultured rat microglia, but not astrocytes. Glia 2004;45(1):75-88.
[46] Verstappen CC, Heimans JJ, Hoekman K, Postma TJ. Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs 2003;63(15):1549-63.
[47] Aluise CD, Sultana R, Tangpong J, Vore M, St Clair D, Moscow JA, et al. Chemo brain (chemo fog) as a potential side effect of doxorubicin administration: role of cytokine- induced, oxidative/nitrosative stress in cognitive dysfunction. Adv Exp Med Biol 2010;678:147-56.
[48] Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev 2002;23(1):90-119.
[49] Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL, et al. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology 2005;30(1):92-100.
[50] Han R, Yang YM, Dietrich J, Luebke A, Mayer-Proschel M, Noble M. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J Biol 2008;7(4):12.
[51] Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA 2000;97(7):3473-8.
[52] Kerb R. Implications of genetic polymorphisms in drug transporters for pharmacotherapy. Cancer Lett 2006;234(1):4-33.
[53] Deprez S, Billiet T, Sunaert S, Leemans A. Diffusion tensor MRI of chemotherapy-induced cognitive impairment in non-CNS cancer patients: a review. Brain Imaging Behav 2013;7(4):409-35.
[54] Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: a review of published studies and recommendations for future research. J Clin Oncol 2008;25(17):2455-63.