Pathogenesis of severe allergic asthma and the therapeutic use of anti- immunoglobulin E antibody
Rukaiya Malik
Tuesday, September 1st, 2015
Allergic asthma involves type 1 hypersensitivity, which is driven by immunoglobulin E (IgE) dependent immunological mechanisms. Severe asthma is associated with chronically persisting inflammation and is often relatively unresponsive to conventional treatment with corticosteroids. This review article summarises the best treatment for severe persistent asthmatics based on current understanding of its pathogenesis. The efficacy and need for the recent therapeutic intervention of anti-immunoglobulin E (anti- IgE) monoclonal antibodies is explored. Further discussion includes drug efficacy and limitations, a summary of cost–benefit analyses, and comparison of anti-IgE to alternative treatment options for asthma. Literature was searched using MEDLINE database to obtain relevant articles. Currently, there is glucocorticoid resistance in certain cases of severe asthma. Hence the viability and safety of anti-IgE antibodies in the treatment of severe asthma was a significant breakthrough. Anti-IgE therapy enhances lung function whilst it reduces number of hospitalisations, frequency of exacerbations and need for inhaled corticosteroids (ICSs). Potential future therapies include monoclonal antibodies against interleukins 5 and 13 (IL-5 and IL-13) for severe asthmatics with persisting eosinophilia. Patients with severe asthma who have become unresponsive to high dose inhaled corticosteroids and who are above the age of six should be prescribed anti-IgE therapy – an effective treatment option that is currently available under the Pharmaceutical Benefits Scheme (PBS).
Introduction
Allergic asthma is a type 1 hypersensitivity reaction that occurs in response to an antigen which would not normally trigger an immune response. Only a small proportion of asthmatics are classified as being severe, yet they contribute to a disproportionately high percentage of health care costs in comparison to mild–moderate asthmatics whose diseases are well-controlled. [1] A cross-sectional study in Barcelona identified that severe asthmatics contributed towards 41% of their total asthma-derived healthcare costs. [1] This review focuses on the pathogenesis of severe allergic asthma, especially the important role of IgE antibodies in the degranulation of mast cells and eosinophils, leading to severe inflammation and contributing to airway remodelling. It also focuses on the mechanism of action and side effects of corticosteroids and anti-IgE antibodies. Glucocorticoid resistance is an important issue in severe asthma and understanding anti-IgE therapy involves having insight into the pathogenesis of allergic asthma. This article also explores the efficacy and limitations of anti-IgE therapy, including a summary of cost–benefit analyses, and a comparison to other options for treatment of asthma.
Pathogenesis
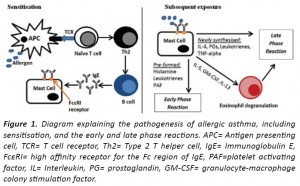
The pathogenesis of asthma involves three major phases (Figure 1): initial sensitisation, and subsequent early and late phase reactions. The initial contact with a particular inhaled allergen is recognised by antigen presenting cells (APCs) such as dendritic cells in the airway tissue. These APCs migrate to bronchus associated lymphoid tissues and lymph nodes and interact with naïve T cells to induce a type 2 T helper (Th2) cell response. Th2 cells stimulate B cell proliferation and isotype switching within germinal centres, resulting in plasma cells switching from producing IgM to IgE antibodies specific to the allergen. [2-4] The Fc portions of IgE antibodies bind to high affinity FcεRI receptors on mast cells and basophils in the submucosa of bronchial tissues, hence completing the sensitisation process. Subsequent contact, when the same allergen binds to and cross-links adjacent Fab portions of IgE molecules present on the surface of mast cells, results in degranulation of the mast cells and release of their mediators. The immediate early phase response involves pre-formed mediators in granules such as histamine being released, which causes bronchoconstriction as well as greater vascular permeability and hence oedema of the bronchial walls and narrowing of the airways. The late phase allergic reaction occurs due to newly synthesised mediators from mast cells such as interleukin 4 (IL-4), prostaglandins, leukotrienes and tumour necrosis factor alpha (TNF-α), which cause infiltration of the bronchial walls with inflammatory cells, especially Th2 cells and eosinophils, leading to increased oedema and airway narrowing. Furthermore, the release of IL-5, granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-13 from mast cells induces the degranulation of eosinophils. These release even more mediators that further perpetuate the condition and can lead to chronic inflammation as seen in severe asthmatics who suffer from frequent exacerbations. Evidently there are serious consequences to the downstream effects of an IgE-mediated response. [5,6]
In the pathogenesis of severe persistent allergic asthma, the activation of IgE that results in Th2-driven chronic inflammation is linked to the development of fibrosis and airway remodelling. [7, 8] The Th2 cytokines, IL-4 and IL-13 along with transforming growth factor beta (TGF-β) increase collagen synthesis and the synthesis of eotaxin which chemically attracts eosinophils. Studies on murine models demonstrate that IL-13 plays a direct role in mucus production. [9] Myofibroblasts that synthesise collagen are responsible for the fibrotic changes seen in airway remodelling in chronic asthma. Other changes seen in airway remodelling include goblet cell metaplasia of airway epithelium and hence increased mucus synthesis and secretion. One possible reason for this could be stimulation by TGF-β. [7] Overall, the resulting disease profile for severe asthma involves persisting symptoms of dyspnoea, coughing and chest tightness, greatly compromised airflow, high eosinophil and Th2 cell differential counts within their full blood count profiles, as well as repeated hospitalisations for severe exacerbations. [10-13]
Management
Inhaled corticosteroids (ICSs)
The current standard treatment for asthma is ICSs, an anti-inflammatory medication, which is often combined with a bronchodilator for symptomatic relief. [10] The mechanism of action of corticosteroids involves binding to the glucocorticoid receptor in the cytosol, which stimulates the receptor to translocate and bind to DNA in the nucleus in order to alter the expression of a variety of genes. [13,14] For instance, corticosteroids inhibit nuclear transcription factor NF-κB and activator protein 1 (AP-1) complex, resulting in decreased production of Th2 pro-inflammatory cytokines. [13-15] Overall, ICSs prevent excessive inflammation involving infiltration by eosinophils and other leukocytes, as well as release of pro-inflammatory mediators that lead to airway remodelling. [13,16]
Even though corticosteroids successfully address the inflammatory consequences of the hypersensitivity reaction, severe asthmatics can become unresponsive to even high doses of ICSs, as well as to oral corticosteroids. The development of glucocorticoid resistance in severe asthmatics is relatively rare but requires appropriate management. [13,17] There are many theories to explain the development of glucocorticoid resistance, including an abnormal interaction between the large amounts of pro-inflammatory mediators and glucocorticoid receptors. [9,18] Hence, in June 2003 the Federal Drug Administration approved the use of omalizumab, the only recombinant human anti- IgE monoclonal antibody (mAb) currently available. [2,16]
Anti-IgE therapy
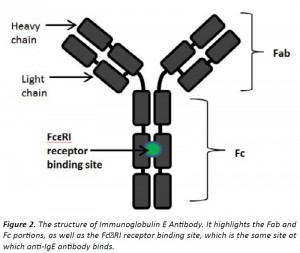
As previously illustrated, IgE antibodies play a crucial role in the pathophysiology of allergic asthma. The synthesis of therapeutically viable anti-IgE mAbs that can target the specific mechanisms of disease pathogenesis is an important breakthrough. [4] Anti-IgE mAb binds to the site on the Fc portion of IgE antibodies that normally binds to FcεRI receptors on mast cells and basophils. [4] Hence once anti-IgE binds to unbound IgE molecules, these IgE antibodies are unable to attach onto mast cells and hence no degranulation and release of inflammatory mediators occurs upon allergen exposure. However anti-IgE is unable to bind to IgE molecules that are already attached to mast cells or basophils due to a conformational change of the Fc portion of IgE once it is bound to the FcεRI receptor on mast cells. [2,4] Consequently, anti- IgE mAb are not able to cross-link IgE on mast cells and basophils and are fortunately non-anaphylactic. [4,8] Circulating anti-IgE:IgE immune complexes are removed by the reticuloendothelial system and do not accumulate in the kidneys, and hence omalizumab has no renal toxicity. [3,16]
Furthermore, complement components do not bind to these immune complexes, no antibodies are produced against anti-IgE, and hence no serum sickness or anaphylaxis occurs. [2,3] This is because the mAb has been carefully manipulated to become “humanised” through the removal of murine components. [4,16] The emphasised safety of the drug is supported by multiple-double blind, randomised control trials (RCT) consisting of greater than 300 participants to compare the effects of omalizumab to placebos in moderate to severe asthmatics. [4,16,19] The reported significant adverse events were primarily injection site reactions. [19,20,21] The drug has only been used for the last eleven years since its approval in the United States and hence the long term side effects are unknown.
Overall, there are many therapeutic benefits of anti-IgE antibodies. These include serum IgE levels diminishing by greater than 95% compared to before treatment and consequently weakening early and late phase reactions. [2,16] Clinical outcomes commonly assessed in trials include the rate of exacerbations, unscheduled healthcare use, asthma-related mortality and quality of life. The 2013 Cochrane review and other systematic reviews identify that omalizumab significantly reduces asthma exacerbations, and specifically that there was a reduction in the rate of exacerbations from 26% to 16% when comparing patients given a placebo to patients receiving omalizumab. [21-23] Similarly, there was a reduction in hospitalisations from 3% to 0.5% when moderate to severe asthmatics were treated with anti- IgE therapy. Furthermore, once treatment with omalizumab begins, patients are more likely to reduce or completely withdraw their use of ICSs, which is further supported by individual RCTs. [19] The RCT conducted by Busse et. al. showed a significant reduction in the number of days with asthma symptoms in comparison to placebo group, a reduction in the need for ICSs as well as a reduction in exacerbations from 48.8% to 30.3% when participants were given omalizumab. [20] Overall, anti-IgE therapy enhances lung function whilst it decreases bronchoconstriction, sputum eosinophilia, hospitalisations, frequency of exacerbations and the need for ICSs. This occurs due to inhibition of the downstream effects of IgE antibodies. [24] Hence anti-IgE mAb provides effective symptom control and improves quality of life.
Current guidelines provided by the National Institute for Health and Care Excellence (NICE) clearly state that omalizumab is recommended as an add-on therapy only for severe persistent asthmatics in individuals above the age of six years old, who are commonly already on high dose ICSs and possibly oral corticosteroids. [25] Safety of omalizumab use among young children has not been determined and therefore there is an age restriction. [20] The pooled analysis of two RCTs involving 1070 moderate–severe asthmatics by Bousquet et al. interestingly showed that patients who had lower lung function or were taking high doses of corticosteroids, or patients who had been hospitalised for asthma treatment in the past year before beginning omalizumab therapy, all displayed the greatest benefit from treatment. [15] Mild to moderate asthmatics would still benefit from anti-IgE therapy; however its use is limited to severe asthmatics primarily due to the large cost of the drug.
The incremental cost effectiveness ratio (ICER) per quality adjusted life year gained for omalizumab is above conventional thresholds – the average annual cost of treatment per patient is £8056 in the UK. [22,25] However the cost effectiveness of omalizumab is justified in severe asthmatics due to their high risk of asthma-related mortality and hence the considerable improvement in quality of life provided by omalizumab. [21,22] Specifically it is reported that severe asthmatics cost the National Health Service (NHS) in the UK approximately greater than £680 million annually. Hence it is subsidised in the UK under the NHS. [25] Initially omalizumab was not PBS-listed until adequate cost–benefit analysis had been conducted. Currently, under Medicare Australia, omalizumab is available under the PBS. [26] However there are strict criteria for satisfying requirements to obtain omalizumab under the PBS. These include having a formal assessment, a corrected inhaler technique, a completed Asthma Control Questionnaire five- item (ACQ-5) and an IgE pathology report. Omalizumab is administered subcutaneously either every two or four weeks, depending on the baseline serum total IgE levels and the patient’s body weight. [26]
Potential future treatment options
Following the successful use and implementation of anti-IgE, there is significant investigation into the efficacy of other mAbs targeting specific inflammatory mediators involved in the pathogenesis of severe allergic asthma. Experimental trials involving monoclonal antibodies against TNF-α and interleukins 4, 17 and 9 (IL-4, IL-17 and IL-9) have not been successful in treating severe allergic asthma. [23,27-29] However mAbs against IL-5 and IL-13 are promising due to their success in trials with reducing frequency of severe exacerbations in patients with severe asthma with persistent eosinophilia. [23,27,30,31] Similar to omalizumab, mAbs against IL-5 such as mepolizumab, relizumab or benzalizumab reduce rate of exacerbations, reduce need for corticosteroids, and improve lung function and asthma control. Clinical trials for use of such immune-modulators have only occurred recently, however it is likely to become a therapeutic option for patients with the specific phenotype of severe asthma with persisting airway eosinophilia. [23,30] Furthermore, several recent studies identify the importance of phenotyping severe asthmatics in order to tailor the most appropriate treatment to each patient. [27,32,33] Personalised treatment will be greatly beneficial for severe asthmatics, however the cost of such endeavours must be considered simultaneously.
It is also important to consider whether there are any currently available alternative treatment options for severe asthmatics. Hence, we shall quickly consider leukotriene receptor antagonists (LTRAs), such as montelukast, and mast cell stabilisers, such as nedocromil. There are several types of leukotrienes (LTs), such as cysteinyl LTs
(CysLTs) and LTB4, and their release plays an important role in the pathogenesis of asthma. Montelukast is specifically a CysLT1 receptor antagonist which does not affect LTB4, an important inflammatory LT in the pathogenesis of airway inflammation in severe asthma. Evidently this drug is not effective in the treatment of severe asthma. [34] As montelukast provides some asthma symptom control, treatment guidelines from the Global Initiative of Asthma (GINA) and the US National Asthma Education and Prevention Program (NAEPP) recommend LTRAs as second-line treatment to ICSs for mild persistent asthma only. [35,36] Nedocromil is a G-protein coupled receptor 35 agonist, which is expressed on human mast cells, and hence causes mast cell stabilisation. [37] This leads to an improvement in lung function and reduces asthma symptoms. Similarly to montelukast, nedocromil only plays a role in mild asthmatics as an alternative treatment to ICSs and there is no current evidence for its role in the treatment of severe asthma. [35,38]
Conclusion
Evidently, severe uncontrollable asthma requires new treatment options other than corticosteroid anti-inflammatory medication due to some patients developing glucocorticoid resistance. Fortunately, numerous randomised control trials have proved the efficacy of anti- IgE therapy for severe asthmatics and now omalizumab is being used clinically. Anti-IgE therapy is particularly effective as it specifically inhibits the IgE-mediated severe inflammatory response which is a critical process in the pathogenesis of allergic asthma. Anti-IgE therapy enhances lung function whilst it reduces number of hospitalisations, frequency of exacerbations and need for ICSs, and greatly improves patient quality of life. It is an effective treatment option that is currently available under the PBS. Potential therapies that may be used in the near future in severe asthmatics with persisting eosinophilia include monoclonal antibodies against IL-5 and IL-13. Future research into reducing the cost of omalizumab and consequently expanding its use for mild–moderate asthmatics would be beneficial.
Acknowledgements
None.
Conflict of interest
None declared.
Correspondence
R Malik: rukaiya.malik@my.jcu.edu.au
References
[1]Serra-Batlles J, Plaza V, Morejon E, Comella A, Brugues J. Costs of asthma according to the degree of severity. European Respiratory Journal. 1998;12(6):1322-6.
[2] Fahy JV, Fleming HE, Wong HH, Liu JT, Su JQ, Reimann J, et al. The effect of an anti-IgE monoclonal antibody on the early-and late-phase responses to allergen inhalation in asthmatic subjects. American journal of respiratory and critical care medicine. 1997;155(6):1828-34.
[3] Brownell J, Casale TB. Anti-IgE therapy. Immunology and Allergy Clinics of North America. 2004;24(4):551-68.
[4] Schulman ES. Development of a monoclonal anti-immunoglobulin E antibody (omalizumab) for the treatment of allergic respiratory disorders. American journal of respiratory and critical care medicine. 2001;164(8 Pt 2):S6-S11.
[5] Wills-Karp M. IMMUNOLOGIC BASIS OF ANTIGEN-INDUCED AIRWAY HYPERRESPONSIVENESS. Annual Review of Immunology. 1999;17(1):255-81.
[6] Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nature medicine. 2012;18(5):693-704.
[7] Gonzalo JA, Lloyd CM, Kremer L, Finger E, Martinez-A C, Siegelman MH, et al. Eosinophil recruitment to the lung in a murine model of allergic inflammation. The role of T cells, chemokines, and adhesion receptors. The Journal of clinical investigation. 1996;98(10):2332-45.
[8] Coyle AJ, Wagner K, Bertrand C, Tsuyuki S, Bews J, Heusser C. Central role of immunoglobulin (Ig) E in the induction of lung eosinophil infiltration and T helper 2 cell cytokine production: inhibition by a non-anaphylactogenic anti-IgE antibody. The Journal of experimental medicine. 1996;183(4):1303-10.
[9] Wenzel S. Mechanisms of severe asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2003;33(12):1622-8.
[10] National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. The Journal of allergy and clinical immunology. 2007;120(5 Suppl):S94-S138.
[11] Moore WC, Clark MP, Dweik RA, Fitzpatrick AM, Gaston B, Hew M, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. The Journal of Allergy and Clinical Immunology. 2007;119(2):405-13.
[12] Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes. The Journal of Allergy and Clinical Immunology. 2004;113(1):101-8.
[13] Poon AH, Eidelman DH, Martin JG, Laprise C, Hamid Q. Pathogenesis of severe asthma. Clinical & Experimental Allergy. 2012;42(5):625-37.
[14] Adcock IM, Ford PA, Bhavsar P, Ahmad T, Chung KF. Steroid resistance in asthma: Mechanisms and treatment options. Current Allergy and Asthma Reports. 2008;8(2):171-8.
[15] Jean B, Sally W, Stephen H, William L, Peter F, Howard F. Predicting Response to Omalizumab, an Anti-IgE Antibody, in Patients With Allergic Asthma. Chest. 2004;125(4):1378-86.
[16] Milgrom H, Fick RB, Su JQ, Reimann JD, Bush RK, Watrous ML, et al. Treatment of Allergic Asthma with Monoclonal Anti-IgE Antibody. The New England Journal of Medicine. 1999;341(26):1966-73.
[17] Reddy D, Little FF. Glucocorticoid-resistant asthma: more than meets the eye. The Journal of asthma : official journal of the Association for the Care of Asthma. 2013;50(10):1036-44.
[18] Adcock IM, Ito K. Steroid resistance in asthma: a major problem requiring novel solutions or a non-issue? Current opinion in pharmacology. 2004;4(3):257-62.
[19] Holgate ST, Thirlwell J, Cioppa GD, Chuchalin AG, Hebert J, Lotvall J, et al. Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clinical & Experimental Allergy. 2004;34(4):632-.
[20] Busse WW, Pongracic JA, Chmiel JF, Steinbach SF, Calatroni A, Togias A, et al. Randomized Trial of Omalizumab (Anti-IgE) for Asthma in Inner-City Children. The New England Journal of Medicine. 2011;364(11):1005-15.
[21] Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. The Cochrane database of systematic reviews. 2014;1:Cd003559.
[22] O’Byrne PM. Role of monoclonal antibodies in the treatment of asthma. Canadian respiratory journal : journal of the Canadian Thoracic Society. 2013;20(1):23-5.
[23] Segal M, Stokes JR, Casale TB. Anti-immunoglobulin e therapy. The World Allergy Organization journal. 2008;1(10):174-83.
[24] National Institute for Healtha and Care Excellence. Omalizumab for treating severe persistent allergic asthma (review of technology appraisal guidance 133 and 201) United Kingdom [cited 2014 June].
[25] Charriot J, Gamez AS, Humbert M, Chanez P, Bourdin A. [Targeted therapies in severe asthma: the discovery of new molecules]. Revue des maladies respiratoires. 2013;30(8):613-26.
[26] Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. American journal of respiratory and critical care medicine. 2013;188(11):1294-302.
[27] Oh CK, Leigh R, McLaurin KK, Kim K, Hultquist M, Molfino NA. A randomized, controlled trial to evaluate the effect of an anti-interleukin-9 monoclonal antibody in adults with uncontrolled asthma. Respiratory research. 2013;14:93.
[28] Haldar P, Brightling CE, Singapuri A, Hargadon B, Gupta S, Monteiro W, et al. Outcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: a 12-month follow-up analysis. The Journal of allergy and clinical immunology. 2014;133(3):921-3.
[29] Piper E, Brightling C, Niven R, Oh C, Faggioni R, Poon K, et al. A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma. The European respiratory journal. 2013;41(2):330-8.
[30] Walsh GM. An update on biologic-based therapy in asthma. Immunotherapy. 2013;5(11):1255-64.
[31] Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. The Journal of allergy and clinical immunology. 2014;133(6):1549-56.