Is switching anticoagulant brands safe – Coumadin and Marevan?
Fiona Chen, Ian Wang, Linda Graudins, Ingrid Hopper
Saturday, June 4th, 2016
Aim: Warfarin is the most frequently prescribed antithrombotic agent, available in Australia as brands Coumadin and Marevan. Although both are manufactured by Aspen Pharmaceuticals, there are differences in formulation. The product information states they cannot be used interchangeably. Two incident reports of warfarin brand interchange in our hospital prompted a literature review. We aimed to review published evidence on the pharmacokinetics and bioequivalence of different warfarin brands and make brand switching recommendations. Methods: Systematic review of the literature on warfarin bioequivalence and incidents reported by the Therapeutic Goods Administration (TGA). Results and discussion: Fifteen studies explored different warfarin formulations. No significant differences were found in efficacy with brand switching in eight studies analysing participants who were healthy, had atrial fibrillation (AF), or a mechanical heart valve. Prospective observational studies demonstrated no significant difference in the International Normalised Ratio (INR) or adverse events, however, a retrospective observational study demonstrated an increase in complications. Of the four population studies, only one demonstrated elevated rates of haemorrhage or thrombosis. No studies directly compared Coumadin and Marevan. Three TGA case reports describe adverse events from brand switching. Conclusion: Studies of different warfarin formulations demonstrate bioequivalence in population studies, but with marked inter-individual variation, hence the recommendation is to continue the same brand of warfarin where possible. However, brand switching is preferable to withholding a dose of warfarin for inpatients, in the absence of the patient’s usual brand. If substituting or brand switching, close monitoring with frequent INR testing is suggested.
Introduction
Warfarin is the most frequently prescribed antithrombotic agent in Australia. Indications include both prevention and treatment of thrombosis, prevention of strokes associated with atrial fibrillation (AF) as well as clotting on mechanical heart valves. [1] Warfarin is also effective in the treatment of deep venous thrombosis and pulmonary embolism. The benefits of warfarin need to be weighed against the risk of haemorrhage, a common complication seen in patients prescribed these anticoagulants. Every year 1.2 to 8.1% of patients on long-term warfarin therapy experience a major bleeding complication, attributable to its narrow therapeutic index, as well as susceptibility to other medications interfering with warfarin’s absorption, metabolism, and clearance. [2,3]
In Australia, there are two brands of warfarin: Coumadin and Marevan. Both brands are now manufactured by the same company, Aspen Pharmacare Australia Pty Ltd, and previously by Boots Healthcare Australia Pty Ltd. [1] Brands differ in tablet colour, markings and excipients, and were marketed before bioavailability testing was required. Prescribing bodies within Australia, including the Therapeutic Goods Administration (TGA) and the National Prescribing Service do not recommend interchanging warfarin brands, Coumadin and Marevan, due to the lack of information on bioequivalence. [1,4] It has been recommended that patients remain on the same brand of warfarin if possible, with more frequent International Normalised Ratio (INR) testing if switching brands. [5] The practice within hospitals in Victoria has been to prescribe Coumadin preferentially, with Marevan prescribed only if the patient has been previously stabilised on this brand. [6]
Two locally reported incidents involving brand switching led to a review of the literature to inform practice. One of the incidents involved a 52-year-old male patient who mistakenly received Coumadin, when Marevan was his usual brand. The nurse administering the nocte medications quickly discovered this incident. The patient’s INR was measured to be sub-therapeutic. The second patient was an 88-year-old female admitted to hospital after a fall and did not receive her regular prescribed Marevan dose, as staff were unable to source that brand after-hours. This patient had an elevated INR prior to the incident and warfarin was subsequently ceased. The two local incidents did not result in change of therapy or result in harm.
We aimed to review published evidence on the pharmacokinetics and bioequivalence of different warfarin brands and make brand switching recommendations.
Methods
Search strategy
A systematic review of the medical literature was performed by FC using PubMed (1996-Jan 2015). Author IW performed a cross-referencing search of Embase (1974-Jan 2015) to ensure the completeness of the literature review. The search terms included warfarin, bioequivalence, Coumadin, and Marevan. Additionally, the search included unpublished reports from the TGA Database of Adverse Events Notification (DAEN), which was searched by author LG for reports of unexpected therapeutic response from substitution of either Marevan or Coumadin. Search parameters on the DAEN: Date Range: 01/01/1971 to 21/05/2014 ‘Therapeutic response unexpected with drug substitution’; Medicine Names: Marevan, Coumadin, Warfarin Sodium. [7] Further unpublished information was included from the Danish Health and Medicines Authority (DHMA).
Eligibility Criteria for Studies
Studies that were considered for inclusion included randomised controlled trials (RCTs), crossover trials, retrospective studies, and case reports, published in English and on human participants, with any follow-up time. Systematic reviews and meta-analyses were analysed but not included. The bibliographies of all retrieved articles were manually checked.
Risk of bias
Risk of bias was assessed in each individual study (Table 1).
Summary measures
Summary measures used include confidence intervals, p-values, and area under the curve (AUC).
Results
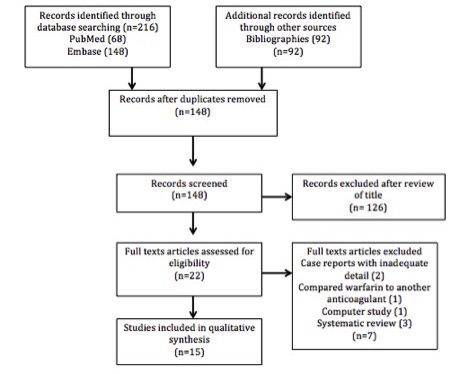
Figure 1: Study flow diagram.
The initial search identified 216 records. After duplicates were removed, 148 records were then screened for eligibility, resulting in 125 articles being excluded after review of the titles. A total of seven full-text papers were excluded for reasons stated in Figure 1.
Fifteen studies were included in the analysis, all published between 1971 and 2011. There were three studies in healthy subjects. There were twelve studies of outpatients taking warfarin, including five RCTs, two prospective observational studies, four retrospective studies and one ecological study. The literature search did not identify any studies in which Coumadin and Marevan (Boots or Aspen) were directly compared.
Pharmacokinetic studies in healthy subjects
Three studies in healthy patients demonstrated no difference in average peak plasma concentrations or AUC, but individual patient variability existed. Müller et al. compared the bioavailability of four warfarin tablet formulations. [8] All products were administered as a single 10 mg oral dose to twelve healthy males, and no significant difference was found in the mean average peak plasma concentrations and AUC (for the plasma concentration time curve). Warfarin bioavailability in the four brands differed by less than 20% when compared to the reference product, Marevan, suggesting that all four types of warfarin could be safely interchanged, without any significant bleeding risk. [8] McGilveray et al. assessed the bioavailability of four warfarin products in eight healthy males. Of these, two sodium warfarin products gave AUC ratios of 97.3% and 100.5% and potassium warfarin of 86.6% relative to the reference products. Significant differences were noted in the time to reach peak concentration, as well as concentration at one hour with one of the products reaching its maximum concentration before this first measurement. [9] Wagner et al. found no significant difference in peak plasma concentration or average plasma concentrations measured at 4, 8, 12, 24, 48, 72, and 96 hours in three different brands of warfarin amongst a group of twelve healthy study subjects. However, there was a statistically significant difference in plasma concentration measured at one hour and in peak plasma concentration time, though it was minimal (p<0.005 and p<0.05 respectively). The three different brands had occurrences of peak plasma concentration at 2.3 hours, 3.6 hours, and 4.1 hours. [10] These studies demonstrate that in healthy males, when brand substitution of warfarin occurred, there was no significant difference in the overall peak concentration of plasma warfarin levels or AUC.
Crossover RCTs
Five RCTs with a crossover design measured the INR of patients with a history of AF or mechanical heart valves who were taking either a branded or generic version of warfarin, having achieved stable anticoagulation with long-term therapy prior to the study. After changing brand, all studies found no statistically significant difference in the average INR of both groups after at least nine weeks of follow-up.
Three studies, Neutel et al., [11] Handler et al., [12] and Weibert et al. [13] examined patients with AF. Neutal et al. demonstrated the bioequivalence of warfarin made by Dupont and Barr Laboratories in a randomised, blinded, crossover study, and showed that the average INR values differed by less than 2% in 55 patients with AF. [11] Handler et al. conducted a study with the objective of substituting warfarin (Dupont) and warfarin (Barr Laboratories) of the same dose and determining its safety and efficacy to confirm bioequivalence results found in other studies. Participants received either warfarin made by Dupont or Barr Laboratories for a period of 28 days and then switched over to the alternate drug for a further two 28-day periods. The mean change in INR measured after every 28 days was -0.17 in patients who began with warfarin (Dupont). After switching, the mean changes were -0.02 and -0.16 respectively. For those starting on warfarin (Barr Laboratories), the mean change from baseline in INR was +0.01, after switching it was -0.16 and -0.18. These differences were not statistically significant, and with the number of dose changes (0.70 ± 0.6 mg for brand A, 0.63 ± 0.9 mg for brand B, and 0.72 ± 0.8 mg for brand C, p=0.89) and mean dose for a stable INR (4.6 ± 2.2 mg, 5.3 ± 2.2 mg, and 5.3 ± 2.4 mg), warfarin (Dupont) was determined to be equivalent to warfarin (Barr Laboratories) in safety and efficacy. Eleven participants were excluded from the study due to dosage adjustments throughout the period, however, an intention-to-treat analysis showed the results fell within the Federal Drug Agency (FDA)’s INR variability values. Handler et al. therefore suggested that additional tests and surveillance are not needed when switching generic with branded warfarin. Weibert et al. performed an RCT crossover comparison of Coumadin and Apothecon warfarin in 19 patients with chronic and paroxysmal AF. [13] Participants already on anticoagulation therapy were randomly assigned to take either Coumadin or Apothecon warfarin for four weeks and then crossed over to receive the alternate for another four weeks. Although seven participants in each group required dosage changes and experienced INR changes outside the desired range, there was no significant change in INR or dosage alteration in either group overall. Therefore, for chronic and paroxysmal AF patients, these brands were considered to be equivalent in anticoagulation action.
Lee et al. studied 35 patients with mechanical valves. [14] This study found no difference in pooled mean INR between Coumadin (Dupont) and warfarin (Lennon) (INR 2.28 and 2.27, respectively) and was within the range for bioequivalence (90% CI for the difference: 96.4 – 104.9). There were also no differences in the adverse event profiles of the two formulations. In a double-blinded crossover RCT, Pereira et al. also suggested that generic and branded warfarin may be used interchangeably. They studied seven patients, and switched them four times between generic Apo-warfarin and to Coumadin over 30 weeks. This study found no significant difference in mean INR results or number of dosage adjustments between patients who switched between the brands and a control group who stayed on Coumadin (p>0.69). They also found no patient and warfarin interaction (p>0.81). [15]
Observational studies
Observational studies were performed by Swenson et al., Milligan et al., and Richton-Hewett et al., observing patient groups switching from brand-name to generic warfarin. Swenson et al. prospectively studied 210 patients, with 105 controls remaining on Coumadin for the study period and 105 patients switching brands. The mean INR difference between the two groups before and after enrolment was not clinically significant (p=0.15). The number of dosage changes required was similar in both groups, with no thromboembolic or haemorrhagic adverse events or any emergency presentations associated with coagulation problems in either group. [16] Milligan et al. prospectively observed 182 participants switching from Coumadin to generic warfarin and demonstrated no significant variation in the parameters studied, including INR (p=0.3), adverse events, and frequency of INR monitoring. [17] One small retrospective study demonstrated an increase in complications with brand switching. Richton-Hewett et al. in 1980 assessed the effect of interchanging brand-name and generic warfarin with a retrospective chart review of 55 patients. [18] The 15 patients who switched to generic warfarin were significantly (p<0.001) more likely to have a prothrombin time (PT) outside of the therapeutic range and were also more likely to require a dosage change. Notably, one patient required a six-day hospital admission with epistaxis and an elevated PT.
Cohort studies
Regional changes in warfarin formulation afforded the opportunity to examine population-based changes in response to brand switching in three cohort studies and one ecological study. These studies demonstrated safety overall, but with some limitations. Halkin et al. observed 975 participants after a nationwide generic switch of warfarin formulations in Israel. [19] They found that INR values were lower and warfarin doses higher (p<0.01), consistent with decreased apparent warfarin sensitivity with the generic brand. Witt et al. found similar results in the United States in a retrospective cohort study of 2299 participants. Calculated INR values were similar, with the average INR decreasing by 0.13 after the switch and no significant differences found in outcomes of hospitalisation, Emergency Department (ED) visits, or bleeding and thromboembolism. However 39% of patients experienced a worsening in therapeutic INR control of more than 10%, whilst 33% experienced INR control that improved by greater than 10%. Witt et al. suggest that receiving long-term anticoagulation therapy with brand-name warfarin can be successfully switched to a generic warfarin, with overall INR levels similar or lower than before such a switch. [20]
A further population-based study was performed by Ghate et al. of 37,756 patients with AF and at least three warfarin prescriptions during the twelve-month follow-up period. The study population was identified via a database of patients receiving commercial health insurance benefits. [19] The patients stayed on Coumadin, generic warfarin, or switched their formulation. An increased risk of thrombotic events was observed in those who switched from Coumadin to generic warfarin compared to those who stayed on Coumadin (hazard ratio (HR)=1.81; 95% CI 1.42 to 2.31, p<0.001). Similarly, increased risk of thrombosis was seen in patients changing from generic warfarin to Coumadin (HR=1.76; 95% CI 1.35 to 2.30, p<0.001) and generic warfarin to another generic type (HR=1.89; 95% CI 1.57 to 2.29, p<0.001). In addition to this, all groups who switched were observed to have an increased risk of haemorrhage. In comparison to the group who remained on Coumadin, switching to the generic formulation was associated with a significantly higher risk of haemorrhagic events (HR=1.51; 95% CI 1.17 to 1.93, p=0.001). [21]
Paterson et al. conducted a population-based, cross-sectional, time series analysis of outpatients aged 66 or older in Ontario, Canada following the province’s switch to one of two generic warfarin formulations (Apo-warfarin and Taro-warfarin) from Coumadin. [22] Trends in warfarin prescribing, INR testing, and hospitalisations for major haemorrhage and stroke were analysed 40 months before, during the one month of, and the nine months after the mandated switch. No significant differences in the rate of INR testing was found (p=0.93), nor hospitalisation for haemorrhage (p=0.97), or cerebral thromboembolism (p=0.89). [22]
Case reports
Exploration of the TGA DAEN for unpublished Australian case reports revealed two adverse drug reactions involving change of warfarin brand, both of which occurred in 2007. They involved an increased INR when the patient changed from Marevan to Coumadin. One of these reports was complicated with a possible drug interaction with concomitant flucloxacillin. The severities of these two reports were not defined. A third report described a patient who experienced an INR decrease when changed from Coumadin to Marevan.
Other case reports have documented adverse events after switching to generic warfarin. The DHMA has made a precautionary decision to cease switching Marevan to generic warfarin Orion after similar adverse drug reaction reports of elevated INR levels. It is important to note that they have not observed any problems with the generic brand and have not ruled out alternate reasons for the changes in INR. [23] In Oklahoma City, two cases saw subtherapeutic INR levels after a switch from Coumadin to generic warfarin despite the Food and Drug Administration asserting bioequivalence. Both reports conclude that INR levels should be closely monitored if switching is unavoidable. [24]
Discussion
This systematic review of the literature identified a number of studies exploring different warfarin brands, using different methodologies and with a variety of measures of bioequivalence in both healthy subjects and patients. However, the brands raising concern in our hospital, Coumadin and Marevan, have not been directly compared. This review demonstrates that there are conflicting findings in individual versus population studies. Overall, there were no significant differences in plasma drug levels or in efficacy as measured by INR with brand switching on an individual patient level. However, population studies demonstrated an increased risk of thrombosis and haemorrhage with brand switching.
Given the widespread use of warfarin, there are a very low number of reports of issues after brand switching both locally and in the published literature. This may be due to under-recognition and under-reporting of adverse reactions. [7] It may also be that brand switching of warfarin rarely occurs and, if it is occurring, then it may be happening without significant adverse events. In Australia, it is recommended that switching warfarin brands should be avoided. Differences in the excipients between the different brands theoretically may affect bioavailability, however, no comparative trials of the Aspen brands have been published. Another important issue in switching brands is the potential for confusion in patients due to different strengths and colours of the medications, potentially resulting in incorrect dosage.
This review demonstrates that when brand switching does occur in a supervised manner, such as that of a clinical trial, the risks are minimal. For hospital inpatients, we would therefore suggest that giving the alternate brand of warfarin is preferable to withholding a dose. Either a return to the patient’s usual brand or a permanent switch to the preferred brand could then be considered. Increased vigilance in following up the INR would be mandatory, due to both the change in formulation and the fact that hospital inpatients are unwell, and that in itself can affect the INR.
The majority of warfarin use, however, is in the community rather than in hospitals. Switching a patient’s warfarin brand should be considered on a case-by-case basis. There should be a compelling reason for brand switching, and the patient’s ability to comprehend the different dosing between brands should be considered. Most importantly, there should be capacity, both by the patient and the pathology provider, to increase monitoring of INR until clinical stability is established on the new brand.
The strengths of this review are that a variety of methods have been used to address the question of safety of switching warfarin brands, which improves the quality of the evidence. With recent updated information available, paired with case examples, it allows a comprehensive expansion on previous reviews exploring similar topics. However, there are several important limitations. The RCTs reviewed all had small sample sizes, with the largest having only 113 participants. [13] This limits the generalisability of these results. Additionally, RCTs may overstate the safety of brand switching due to selection bias, for example, (1) patients on anticoagulation who agree to enter a trial may manage their anticoagulation better than the general population, (2) well-controlled patients are more likely to participate in a trial, or (3) compliance with medication and dietary restrictions increases during the period of the trial. Studies focusing on pharmacokinetics and bioequivalence may not be adequate to assess clinical outcomes. There are also biases in population studies. For example, factors such as changes to the environment affecting the whole region and contributing to the outcome may not be recognised. Ecological studies cannot be used to prove or explore associations between determinants of disease. There are limitations in the use of a retrospective dataset, such as health insurance claims, in which it cannot be confirmed whether individuals actually took the warfarin dispensed by the pharmacy. Additionally, if the dataset was created for an alternate purpose, such as discharge coding to determine reimbursement, inconsistent definitions, or coding practices may mean the data is of too poor quality to use in a study. There may also be inadequate information about the confounders and data quality. Differential loss to follow-up can introduce bias in retrospective and prospective cohort studies. Across all studies, publication bias and selective reporting within the studies may influence the cumulative evidence.
Conclusion
Studies of different brands of warfarin demonstrate that brand switching is generally safe, although some population studies have demonstrated that INR control may worsen for a period, and that risk of both thrombosis and haemorrhage may increase. Based on the studies reviewed, we support the recommendation to continue with the same brand of warfarin, if possible. However, in the inpatient hospital setting, brand switching is preferable to withholding a dose of warfarin in the absence of the preferred brand, with appropriate INR testing afterwards. We therefore recommend that in an in-patient situation, substitution with an alternate brand of warfarin should occur if one brand is unavailable and INR is monitored daily until stability is assured. In the community, brand switching should be considered on a case-by-case basis with increased INR monitoring until clinical stability is reached. Phasing out formulations of warfarin so only one brand is available, with support from the local regulatory drug agency, including advisory information and support for patients and clinicians, would resolve the confusion.
Conflicts of Interest
None declared.
References
[1] Product Information: Marevan, Coumadin. Aspen Pharmacare Australia Pty Ltd. eMIMS (accessed 5/7/2015).
[2] Levine MN, Raskob G, Landefeld S, Kearon C. Hemorrhagic complications of anticoagulant therapy. Chest. 1998;114:511S-523S.
[3] Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ, Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, Chest. 2012;141:1412S3.
[4] Adverse Drug Reactions Advisory Committee, Coumadin and Marevan are not interchangeable, Australian Adverse Drug Reactions Bulletin, June 1999;18:2.
[5] Fry FK, PBAC, Boots. Warfarin tablets, Aust Prescr 1997;20:33.
[6] Dooley M. Recommendations for warfarin in Victorian public hospitals [letter]. Aust Prescr 2003;26:27-9.
[7] Adverse Drug Reaction Reports. Received via personal communication with the Therapeutic Goods Administration. 4th April 2014.
[8] Müller FO, Steyn JM, Hundt HK, Luus HG (1988 Dec 3) Warfarin bio-availability. A comparison of 4 products. S Afr Med J, 74, pp. 1.
[9] McGilveray IJ, Midha KK, Cooper JK: Bioavailability of Canadian tablet formulations of Warfarin Sodium and Potassium. Can J Pharm Sci. 1978;13:9-11.
[10] Wagner JG, Welling PG, Lee KP and Walker JE: In vivo and in vitro availability of commercial Warfarin tablets. J Pharm Sci. 1971;60:666-677.
[11] Neutel JM, Smith DHG. A randomized crossover study to compare the efficacy and tolerability of Barr warfarin sodium to the currently available Coumadin. Cardiovasc Rev Rep. 1998;19:49–59.
[12] Handler J, Nguyen TT, Rush S, Pham NT. A blinded, randomized, crossover study comparing the efficacy and safety of generic warfarin sodium to Coumadin. Prev Cardiol. 1998;4;13–20.
[13] Weibert RT, Yeager BF, Wittkowsky AK, Bussey HI, Wilson DB, Godwin JE et al. A randomized, crossover comparison of warfarin products in the treatment of atrial fibrillation. Ann Pharmacother. 2000;34:981–8.
[14] Lee HL, Kan CD, Yang YJ. Efficacy and tolerability of the switch from a branded to a generic warfarin sodium product: an observer-blinded, randomized, crossover study. Clin Ther. 2005;27:309–19.
[15] Pereira JA, Holbrook AM, Dolovich L, Goldsmith C, Thabane L, Douketic JD et al. Are brand-name and generic warfarin interchangeable? Multiple n-of-1 randomized, crossover trials. Ann Pharmacother. 2005 Jul-Aug; 39:1188-93.
[16] Swenson CN, Fundak G. Observational cohort study of switching warfarin sodium products in a managed care organization. Am J Health Syst Pharm. 2000;57:452-455.
[17] Milligan PE, Banet GA, Waterman AD, Gatchel SK, Gage BF. Substitution of Generic Warfarin for Coumadin in an HMO Setting. Ann Pharmacother. 2002;36: 764-768.
[18] Richton-Hewett S, Foster E, Apstein CS: Medical and economic consequences of a blinded oral anticoagulant brand change at a municipal hospital. Arch Intern Med. 1998;148:806-808.
[19] Ghate SR, Biskupiak JE, Ye X, Hagan M, Kwong WJ, Fox ES, et al. Hemorrhagic and thrombotic events associated with generic substitution of warfarin in patients with atrial fibrillation: a retrospective analysis. Ann Pharmacother. 2011;45: 701-12.
[20] Halkin H, Shapiro J, Kurnik D, Loebstein R, Shalev V, Kokia E. Increased warfarin doses and decreased international normalized ratio response after nationwide generic switching. Clin Pharmacol Ther. 2003;74:215-221.
[21] Witt DM, Tillman DJ, Evans CM, Plotkin TV, Sadler MA. Evaluation of the clinical and economic impact of a brand name-to-generic warfarin sodium conversion program. Pharmacotherapy. 2003;23:360-368.
[22] Paterson JM, Naglie G, Laupacis A, Stukel T. Clinical consequences of generic warfarin substitution. JAMA. 2006;296:1969-1972.
[23] Danish Health and Medicines Authority. Warfarin Orion must not replace Marevan and Waran. Danish Pharmacovigilance. 2015[cited 2015 July 4]. Available from: https://sundhedsstyrelsen.dk/en/news/2015/danish-pharmacovigilance-update,-april-2015.
[24] Hope KA1, Havrda DE. Subtherapeutic INR values associated with a switch to generic warfarin. Ann Pharmacother. 2001 Feb;35:183-7.
Table 1: Summary of studies
| Reference
Year |
Country | Trial design* | Participants | Number of subjects | Follow up (weeks) | Mean age (years) | Males (%) | Brands | Outcomes | Limitations and bias |
| Müller8 1988 | South Africa | RCT | Healthy males | 12 | 1.14 (8 days) | 22 | 100 | Warfarin (Petersen), Warfarin (Lennon), Coumadin (Boots),
Marevan (Allen & Hanburys) |
Test products differed less than 20% from Marevan | Single blind – experimenter’s bias
Small study size Only male participants |
| McGilveray9 1978 | Canada | Crossover trial | Healthy males | 8 | None | 25-42
(range) |
100 | Sodium warfarin
Potassium warfarin (Manufacturers not stated) |
No difference in average peak plasma concentrations. Significant difference in the time required to reach peak concentrations. Two sodium warfarin products gave AUCs of 97.3% and 100.5%. The potassium warfarin gave a lower value of 86.6% relative to the reference products | No mention of blinding – performance bias
No mention of randomisation – selection bias Small study size Only male participants |
| Wagner10 1971 | US | Crossover trial | Healthy subjects | 12 | 1 | 25 | 83 | Warfarin (3 different manufacturers not identified) | No overall significant difference in average peak plasma concentrations or average time taken to reach peak plasma concentration. A small statistically significant difference in plasma concentration measured at 1 hour and in peak plasma concentration time (p<0.005 and p<0.05 respectively) | No mention of blinding – performance bias
No mention of randomisation – selection bias Small study size Study performed more than 44 years ago |
| Neutel 11
1998 |
SB crossover RCT | Outpatients Atrial Fibrillation | 39 | 9 | 70 | 100 | Coumadin (Dupont) Warfarin (Barr Laboratories) | Changes in INR after switching were not significant (p>0.05); no differences in adverse effect profiles | AF participants – detection bias
Only male participants Small study size |
|
| Handler 12
1998 |
US | DB crossover RCT | Outpatients Atrial Fibrillation | 57 | 12 | 69 | 65 | Coumadin (Dupont) Warfarin (Barr Laboratories) | No significant differences in INR (p=0.40), dose adjustments, adverse events | AF participants – detection bias
Small study size Funded by generic manufacturer |
| Weibert13
2000 |
US | DB crossover RCT | Outpatients Atrial fibrillation | 113 | 14 | 70 | 75 | Coumadin (DuPont) Warfarin (Apothecon) | No significant differences in daily dose (0.5 mg/d), average INR difference (< 0.08), adverse events | AF participants – detection bias
Small study size Single blind of investigators not patients – performance bias Increased regularity of INR monitoring, which may have detected more variability than normal Funded by generic manufacturer |
| Lee14
2005 |
Taiwan | SB crossover RCT | Mechanical heart valves | 35 | 12 | 52 | 71 | Coumadin (Dupont) Warfarin (Lennon) | Dose changes were rare; no significant differences in pooled INRs between Coumadin (Dupont) and warfarin (Lennon) (INR 2.28 and 2.27, respectively). The 90% CI for the difference was 96.4 – 104.9 | HV participants – detection bias
Observer blinded Small study size |
| Pereira15
2005 |
US | DB crossover RCT | Outpatients Various indications | 7 | 30 | 63 | 43 | Coumadin (Bristol-Myers Squibb) Warfarin (Apotex) | No significant differences in mean INR measurements or variation (p>0.69). No patient and warfarin interaction found (p>0.81) | Small study size
Patients underwent dosage adjustments if INR was out of target range |
| Swenson16
2000 |
US | Prospective observational cohort study | Outpatients Various indications | 210 | 20 | 78 | 50 | Coumadin (Dupont) Warfarin (Barr Laboratories) | No significant differences in INR between groups (p=0.15); no adverse effects or events | Non-randomised assignment – selection bias
Confounding |
| Milligan17
2002 |
US | Prospective observational study | Outpatients Various indications | 182 | 78 | 75 | 57 | Coumadin (Bristol-Myers Squibb) Warfarin (Barr Laboratories) | No significant differences in INR (p=0.3), dose adjustments, adverse events | Funded by an insurance company
Non-randomised assignment – selection bias Confounding |
| Richton-Hewett18
1998 |
US | Retrospective cohort study | Outpatients Various indications | 55 | 30 | 56 | 47 | Coumadin (Du Pont) Panwarfarin (Abbott Laboratories) | Higher rate of INR out of range (p<0.001), dose changes (p<0.05), clinic utilisation (p<0.03) with generic group; no significant differences in morbidity/mortality | Non-randomised assignment – selection bias
Non-blinded staff – experimenter’s bias Confounding Small study size |
| Halkin19
2003 |
Israel | Retrospective observational study | Outpatients Various indications | 975 | 52 | 70 | 47 | Coumadin Sodium (Taro Pharmaceutical Industries)
Coumadin Sodium clathrate (Taro, new formulation) |
After the switch, INR values were lower and warfarin doses prescribed were higher (p<0.01) | Non-randomised assignment – selection bias
Confounding Data from administrative database |
| Witt20
2003 |
US | Retrospective cohort study | Outpatients Various indications | 2299 | 26 | 69 | 54 | Coumadin
Warfarin (Barr Laboratories) |
INR values below therapeutic range with generic (p<0.0001); overall average INR decreased by 0.13 after switch; no significant differences in hospitalisations, ED use, outcomes (bleeding or thromboembolism) | Non-randomised assignment – selection bias
Confounding Fatal adverse effects not included in study as post-conversion questionnaire required |
| Ghate21
2011 |
US | Historical cohort analysis | Atrial fibrillation | 37 756 | 52 | 71 | 58 | Generic warfarin Coumadin (DuPont/Bristol-Myers Squibb) | Increase in thrombotic events in those groups who had switched
Coumadin to generic warfarin (HR=1.81; 95% CI 1.42 to 2.31, p<0.001) Generic warfarin to Coumadin (HR=1.76; 95% CI 1.35 to 2.30, p<0.001) Generic warfarin to another generic (HR=1.89; 95% CI 1.57 to 2.29, p<0.001) Increase in haemorrhagic events in those groups who had switched to generic (HR=1.51; 95% CI 1.17 to 1.93, p=0.001) |
Non-randomised assignment – selection bias
Information from insurance claims database with no available information about INR monitoring, adherence to therapy and missed follow-up appointments Confounding |
| Paterson23
2006 |
Canada | Ecological study | Outpatients Various indications | 36 724 | 200 | >65 years old | Not specified | Coumadin (Bristol-Myers Squibb) Warfarin (Apo-warfarin and Taro-warfarin) | No significant differences in INR testing (p=0.93) or hospitalisation for haemorrhage (p=0.97) or thromboembolism (p=0.89) | Funded by the government
Focussed on major clinical events needing hospitalisation but excluded other outcomes with less reliable code, for example, DVT Cross-sectional – cannot determine causation Administrative health data used |
