Assessing cardiac output in the perioperative patient
Miss Alexandra Richards
Sunday, September 25th, 2016
Cardiac output (CO) is an essential component in the evaluation of the critically unwell hospitalised patient’s physiological state. As an estimated measure of cardiac function, CO is of high clinical importance to determine how well nutrients and oxygen are delivered to body tissue. Additionally, as its determinants are related to circulating volume and heart rate, it can be used as a surrogate measure for any homeostatic imbalances, which may require critical medical intervention. This article compares the available clinical measurements of CO. The Pulmonary Artery Catheter (PAC) remains the most accurate and reliable method, however is a highly invasive measure. Minimally invasive techniques reduce the risk of procedural complications, but do so at the expense of reliability. Of these methods, pulse contour analysis is the most extensively studied, with precision being similar, if not equivalent to, PAC. However, until definitive, outcome-based, comparison studies have been completed, the selection of the most appropriate CO measurement modality remains the decision of the treating clinician, the patient and relevant clinical guidelines.
Introduction
When assessing critically unwell hospitalised patients, haemodynamic monitoring is an important indicator of the patient’s condition. Cardiac output (CO) assessment is an essential component of the patient’s physiological state during their perioperative period. CO is an estimated measure of cardiac function calculated by multiplying the heart rate (beats per minute) by stroke volume (volume of blood pumped out of the heart in mL) [1]. CO is of high clinical importance, as it is one of the determinants of how well nutrients and oxygen are delivered to body tissue, with a normal CO defined as 4-8 L/min in healthy individuals, varying with gender and body habitus [1,2]. Additionally, as its determinants are related to circulating volume and heart rate, it can be a surrogate measure for any homeostatic imbalances (such as haemorrhage and volume depletion or sympathetic activation in stress raising heart rate) that may indicate the need for critical medical intervention.
Importantly, CO is also a dynamic way to assess organ perfusion and cardiac function intraoperatively, in addition to providing an indication of likely expected outcomes and complications postoperatively [1]. Other important clinical aspects of care to assess and monitor include the assessment of end organ function. This includes conscious state, respiratory rate, blood pressure, peripheral perfusion (temperature and capillary refill time), urinary output, and markers of metabolic acidosis [1,3].
Multiple invasive, semi-invasive, and non-invasive methods of assessing CO in the clinical setting are available. Five common and emerging methods are summarised and compared below (Table 1).
| Method | Principle | Advantages | Disadvantages | |
| Invasive | Pulmonary artery catheter
(PAC) |
Uses the Stewart-Hamilton equation: the rate of blood flow is inversely proportional to the change in temperature over time. |
|
Risk of:
|
| Non-invasive | CO2 rebreather | Uses the Fick principle: the conservation of mass, which allows the calculation of blood flow to an organ based on the uptake of a specific marker substance. |
|
|
| Aortic and echocardiography Doppler ultrasound | Doppler ultrasound operates on the principle that the shift in frequency of a wave between two points is directly proportional to the velocity of that wave. |
|
|
|
| Bio-impedance | Measures electrical impedance of the thoracic cavity generated during systole and left ventricular outflow into the aorta. The ratio of applied current and measured voltage equals the bio-impedence, which is measured over time. |
|
|
|
| Semi-invasive | Pulse contour analysis | Based on the hydraulic principle between flow, pressure, and time; measures pulse pressure (the difference between diastolic and systolic pressure) as proxy to volume, in order to create a picture or “wave form” that can be analysed mathematically to find stroke volume |
|
|
Invasive methods: pulmonary artery catheter
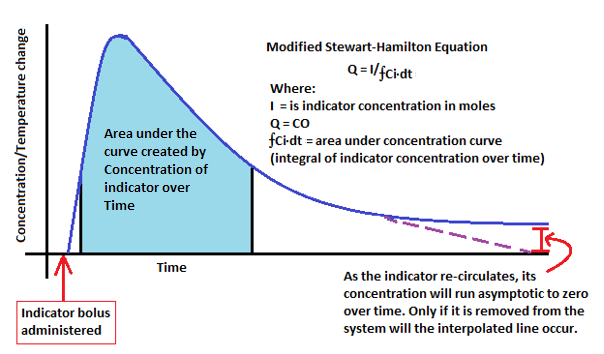
The pulmonary artery catheter (PAC) was introduced in 1970 by Harold Swan and is often used as the gold standard for CO monitoring [1]. This technique involves the insertion of a catheter, preferably through the right internal jugular vein because of ease of insertion, proximity to the heart’s right atrium, and rarity in anatomical variation between patients in this vein, although other sites can be used, particularly the subclavian veins. The device has an inflatable balloon at its tip, which permits it to be floated through the right cardiac chambers and into the pulmonary artery. The PAC estimates CO using a technique called thermo-dilution. This involves administration of a bolus of 10 mL of saline (0.9% NaCl at room temperature) injected into the right atrium via a proximal catheter port. The difference in temperature is measured through the thermistor (thermally sensitive probe) on the PAC’s tip [1]. From this, a CO value is calculated using the Stewart-Hamilton equation. This equation is based on the principle that the rate of blood flow is inversely proportional to the change in temperature over time (the concentration of the indicator solution divided by the “area under the curve” or integral created by the indicator solution concentration change over time) (Figure 1) [2]. Such a reading can either be continuous or not depending on both the requirement of the clinical setting and the form of PAC [4].
Figure 1: Modified Stewart-Hamilton equation applied in PAC Thermal dilution during CO monitoring [2].
The use of the PAC method for CO monitoring has many advantages over other techniques. Conversely, due to it being an invasive device, it does carry inherent risks including an increased possibility of dysrhythmias such as complete heart block, perforation of heart chambers, cardiac tamponade, pneumothorax, valve damage, infection, and emboli [1]. Post hoc analyses of larger studies have also reported no benefit in using the PAC method, other than in elective surgical patients [5]. However, given that elective surgical patients are often healthier than non-elective intensive care patients, queries over confounding factors within the population have been raised [5]. This demonstrates that it can be used safely, but brings into question its patient-related value in situations arising from the critical care environment [6]. Despite the relatively high reliability of this device, debate still exists around whether it actually improves outcomes in various patient groups [6]. Regardless, its clinical benefit in monitoring both undifferentiated, multi-factorial shock states and cardiac cases is well documented [6].
Minimally invasive methods: CO2 re-breather, Doppler ultrasound, and bio-impedance
Indirect Fick principle (CO2 re-breather)
The pulmonary circulation is the part of the cardiovascular system that involves deoxygenated blood flowing from the right heart, through the lungs, and back into the left side of the heart (now oxygenated blood). This circulation can be used to estimate CO via the use of a mathematical equation called the “Fick” principle [7]. The Fick principle is a mathematical interpretation of gasses, based on the conservation of mass, which allows the calculation of blood flow to an organ based on the uptake of a specific marker substance [2]. In more clinical terms, it grants the ability to observe the amount of gas release, classically oxygen (O2) or carbon dioxide (CO2), that occurs in the pulmonary circuit, via the alveolar blood flow, and the difference in gas concentration in the arterial and venous circulation (by collecting venous and arterial blood samples) [7]. By measuring alveolar blood flow via the rate of carbon dioxide volume (VCO2) produced from the lungs and body over the arterio-venous carbon dioxide gradient of arterial carbon dioxide partial pressure (PaCO2) and venous carbon dioxide partial pressure (PvCO2), one can extrapolate CO from the difference between these CO2 gradients [8]. Mathematically, with Q representing CO and VO2 being volume of oxygen consumed, the generic formula is as follows:
VO2 = Q (PaCO2 – PvCO2) [8]
For example, if the concentration of CO2 being delivered to an organ is known, and the amount this concentration increases after perfusing the organ (venous and arterial difference) is also known, it is possible to divide the two in order to determine the “flow” of gas, and therefore blood, to that organ [7]. In CO monitoring, the use of the pulmonary circuit to represent “the body”, with a sample taken before (venous) and after (arterial) allows us to determine the change in CO2 concentration as the blood flows to the heart. Concurrently, the patient in which CO is being measured must be intubated and under mechanical ventilation in order to control this gas exchange and its volume parameters [8].
The advantages of using the Fick technique are largely associated with it being a minimally invasive technique (such as relatively fewer contraindications and adverse outcomes than comparable methods) [7]. However, this method has limitations in that it has a lower level of accuracy than invasive methods, and requires the patient to undergo intubation [7]. Additionally, it was found that in the determination of CO for patients undergoing cardiac surgery, an underestimation preoperatively and an overestimation postoperatively commonly occurred [9]. This brings into question its clinical reliability in the vital perioperative setting [9]. Thus, although it has clinical advantages in patients who cannot tolerate more invasive methods, its reliance clinically is not as consistent as that of PAC.
Doppler ultrasound: aortic and echocardiography
Doppler ultrasound operates on the principle that the shift in frequency of a wave is directly proportional to the velocity of the moving plasma [10]. By measuring plasma velocity, Doppler ultrasound can be used to estimate CO. Typically, Doppler ultrasound calculates CO by measuring the velocity of blood plasma at the level of the thoracic aorta, generating an estimation of blood flow (stroke volume) by measuring plasma flow across the cross-sectional area of the aortic vessel or valve. This velocity can then be multiplied by the heart rate to provide an estimation of CO [7]. This technique is commonly combined with echocardiography, which measures left-side cardiac filling pressures via a two-dimensional ultrasound, and Doppler measurement of the aortic annulus diameter [11]. Additional benefits derived from this method include the provision of clinically relevant information on the global structure of the heart, in addition to valvular anatomy and presence of pericardium pathology (including tamponade or constrictive pericarditis) [11]. This allows for accurate, non-invasive measurement of left ventricular diastolic dysfunction, which is predictive of mortality in hospitalised patients [10]. Furthermore, echocardiography can also measure the response of stroke volume to both fluid bolus and diuretic therapy, while monitoring left (trans-mitral) and right (vena cava) arterial pressure [12]. This may be critical in patients at risk of both systolic and diastolic heart failure [12]. Importantly, these changes can be monitored serially after interventions have been performed.
Significant drawbacks of this method include its inability to provide continuous monitoring and difficulty in measuring sample volume placement; probe placement and beat-to-beat variation in stroke volume both impact accuracy [11]. Additionally, in the setting of decompensated systolic heart failure, tissue Doppler imaging can be inaccurate for monitoring filling pressures [11]. Since this technique also requires particular placement of the probe, as this is an important variable for reproducible results, significant operator training and skill is required [12]. Therefore, while this method is both reliable and has multiple reasons for use in the clinical setting, consideration needs to be given to operator skill and reproducible monitoring. An additional limitation is that echocardiography requires considerable skill to perform well.
Finally, while not commonly used in Australia, oesophageal and transthoracic Doppler methods also exist. As with Doppler echocardiography, monitoring has a similar level of reliability as more invasive methods, but is predicated upon three factors: the aortic cross-section accuracy, the parallel placement of the transducer, and the maintenance of constant beam direction between measurements [7]. Even controlling for these variables, inherent factors limit this technique, including physiological expansion of the aorta during systole, noted to be approximately 12% [10]. Furthermore, the use of a nomogram (a pre-determined graph using three or more logarithmic scales to show a relation between them, such as occurs in weight and height charts to calculate body mass index) introduces potential measurement error secondary to variation with vascular tone and volume status that needs to be considered for all measurements [10].
Bio-impedance
CO can also be estimated via bio-impedance in a technique known as impedance cardiography. This technique measures the electrical impedance of the thoracic cavity generated during systole and left ventricular outflow into the aorta through the conductive properties of blood via four electrodes placed around the thorax; two placed on the left neck and another two upon the lower thorax, focus alternating current toward the ascending and descending aorta, within which blood is the most conductive material [13]. Put simply, the electrodes emit a small electrical current that is then “bounced” off conductive tissue, of which blood produces the strongest “bounce”. This current is then received by the electrode, wherein the change of impedance (current “bounced” from the blood tissue and received by the electrode) correlates to stroke volume [1].
Similar to all minimally invasive techniques, impedance cardiography nullifies the risk of infection and haemorrhage, however, it remains particularly sensitive to movement and is unsuitable for use in patients with arrhythmias or who are haemodynamically unstable [7]. This limits its use in Australian medical settings and so it is not commonly seen in practice [7,13].
Semi-invasive: pulse contour analysis
The final, commonly used method of estimating CO is pulse contour analysis. Pulse contour analysis utilises arterial waveforms, obtained either from an arterial catheter (invasive) or a peripheral finger probe (minimally invasive), in order to extrapolate stroke volume and systemic vascular resistance [2]. This technique relies on measuring pulse pressure, that is, the difference between diastolic and systolic pressure (as proxy to volume), in order to create a picture or “wave form” that can be analysed mathematically to find the area under the curve. Initially, this method assumed compliance of aortic wall to be uniform, regardless of patient demographics and co-morbidities (as the compliance of a vessel alters its distensibility). However, via the use of an algorithm developed by Wesseling et al. [14], it become possible to gauge stroke volume by integrating the area under the curve measured during systole to minimise this error. Concurrently, the estimated stroke volume can then be multiplied by heart rate to give CO [7,14,15]. Many devices rely on the principle of pulse contour analysis, such as Pulse Contour Cardiac Output Monitoring (Pulsion Medical Systems, Munich, Germany) and Lithium Chloride Dilution (LiDCO Ltd., Cambridge, UK), are invasive, needing arterial lines. However, the Vigileo™/FloTrac™ system (Edwards Lifesciences, Irvine, CA, USA) and the Nexfin® system (BMEYE, Amsterdam, Netherlands), which uses a finger cuff to measure pulse pressure of the digital arteries, are non-invasive options. Currently, all of these devices are limited by the fact that they require inter-patient calibration [16].
As in the bio-impedance method, this technique is limited in patients with cardiac arrhythmias, aortic regurgitation and intra-aortic balloons as they alter measurement accuracy [4]. Equally, the use of arterial catheters introduces an additional risk of infection and haemorrhage. Despite this, a recent meta-analysis performed by Mayer et al. determined that a strong positive correlation exists between the contemporary pulse contour analysis devices (FloTrac/Vigileo) and the PAC method when measuring CO [16]. Concurrently, this method possesses a high clinical applicability with significant potential for future development.
In conclusion, accurate CO assessment and monitoring in the perioperative patient remains critically important. While PAC remains the most accurate and reliable method, its invasiveness and the subsequent risk of complications makes it unsuitable for specific patient subsets. Conversely, while minimally invasive techniques reduce the risk of procedural complications, this is often at the expense of reliability. Of these methods, pulse contour analysis is the most extensively studied, with precision being similar, if not equivalent to, PAC. Until definitive, outcome-based comparison studies have been completed, the selection of the most appropriate CO measurement modality remains the decision of the treating clinician, the patient, and faculty clinical guidelines.
Acknowledgements
Dr M. Peach.
Conflicts of Interest
None declared.
References
[1] Wigfull J, Cohen AT. Critical assessment of haemodynamic data. Contin Educ Anaesth Crit Care Pain. 2005 Jun 1;5(3):84-8.
[2] Scheer BV, Perel A, Pfeiffer UJ. Clinical review: complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Crit Care. 2002 Apr 18;6(3):199.
[3] Calzia, E, Iványi, Z, Radermacher P. Functional haemodynamic monitoring. Berlin: Springer Berlin Heidelberg; 2005. Determinants of blood flow and organ perfusion; p.19-32.
[4] Lavdaniti M. Invasive and non-invasive methods for cardiac output measurement. Int J Caring Sci. 2008 Sep 1;1(3):112.
[5] Harvey SE, Welch CA, Harrison DA, Rowan KM, Singer M. Post hoc insights from PAC-Man—the UK pulmonary artery catheter trial. Crit Care Med. 2008;36(6):1714-21.
[6] Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, Elbourne D et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet. 2005 Aug 6;366(9484):472-7.
[7] Drummond KE, Murphy E. Minimally invasive cardiac output monitors. Contin Educ Anaesth Crit Care Pain. 2011 Oct 3:mkr044.
[8] Chaney JC, Derdak S. Minimally invasive hemodynamic monitoring for the intensivist: current and emerging technology. Crit Care Med. 2002 Oct 1;30(10):2338-45.
[9] Alhashemi JA, Cecconi M, Hofer CK. Cardiac output monitoring: an integrative perspective. Crit Care. 2011 Mar 22;15(2):214.
[10] Dark PM, Singer M. The validity of trans-esophageal Doppler ultrasonography as a measure of cardiac output in critically ill adults. Intens Care Med. 2004 Nov 1;30(11):2060-6.
[11] Porter TR, Shillcutt SK, Adams MS, Desjardins G, Glas KE, Olson JJ et al. Guidelines for the use of echocardiography as a monitor for therapeutic intervention in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiog. 2015;28(1),:40-56.
[12] Konstadt SN, Shernan SK, Oka Y, editors. Clinical transesophageal echocardiography: a problem-oriented approach. Philadelphia: Lippincott Williams & Wilkins; 2003.
[13] Mehta Y, Arora D. Newer methods of cardiac output monitoring. World J Cardiol. 2014 Sep 26;6(9):1022.
[14] Wesseling KH, Jansen JRC, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using non-linear, three element model. J Appl Physiol, 1993; 74:2566-73.
[15] Mathews L, Singh KR. Cardiac output monitoring. Ann Card Anaesth. 2008 Jan 1;11(1):56.
[16] Mayer J, Boldt J, Poland R, Peterson A, Manecke GR. Continuous arterial pressure waveform–based cardiac output using the FloTrac/Vigileo: a review and meta-analysis. J Cardiothor Vasc An. 2009 Jun 30;23(3):401-6.