Is cellular senescence a viable strategy and endpoint for oncological control?
Kok-Ho Ho
Tuesday, December 1st, 2015
Apoptosis is considered the main form of cell death in cancer cells undergoing cytotoxic treatments such as chemotherapy and radiotherapy. However, disappointing treatment response rates in some cancers have prompted a rethink regarding oncological control methods. Cellular senescence has emerged as a possible tumour suppression strategy that may effectively control cancer cells when apoptosis fails. Understanding the mechanistic workings of senescence in the context of cancer cells may shed light on its feasibility as a clinical strategy.
Conventional cancer therapeutics such as chemotherapy rely heavily on cytotoxicity to achieve maximal cell death. The rationale behind this approach is that elimination of cancer cells, and consequently tumour burden, will help achieve the best clinical outcome. Induction of cell death as an immediate clinical endpoint might be seen as an obvious choice, but it is worth contemplating whether this short-term benefit is incurred at the expense of long-lasting remissions. Many of the anti-cancer agents used in chemotherapy activate DNA damage signaling pathways which lead to apoptosis. However, apoptotic pathways tend to be defective in cancer cells. This could explain why response rates are sub-optimal despite aggressive regimens. The continued use of cytotoxic agents also promotes the development of resistant clones which can repopulate the primary tumour or metastasise to distal sites. If cell death is not always the best way to achieve sustainable cancer control, is there an alternative strategy or endpoint that overcomes the subversion of apoptotic cell death by tumour cells and is just as effective in blunting their proliferative nature?
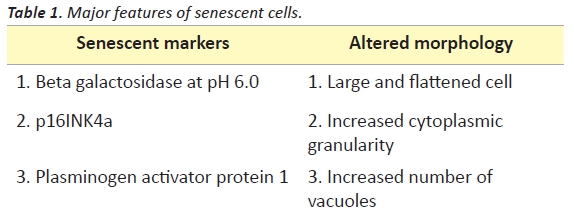
One possible answer seems to be the induction of cellular senescence. Cellular senescence is classically defined as an irreversible state of growth arrest that occurs when cells encounter stress stimuli. Senescent cells are characterised by the following major features (Table 1).
Different forms of cellular senescence such as replicative (i.e. due to telomere shortening) or oncogene-induced senescence exist. Senescence-like phenotypes can be rapidly induced by genotoxic stress imposed by chemotherapy or radiotherapy, also known as accelerated cellular senescence (ACS). [1] Both apoptosis-competent and apoptosis-defective cancer cells may still be controlled by senescence, therefore implicating it as an important tumour-suppressive mechanism. [2] However, chemotherapy may not always provide durable responses as subsets of cancer cells are capable of escaping senescence and resuming cell division. The utility of senescence has, thus, remained inconclusive. This article will attempt to briefly explore the mechanistic insights of cellular senescence in cancer cells and assess its feasibility as a clinical strategy or endpoint in oncological control.
Mechanistic insights into the role of cellular senescence in cancer cells
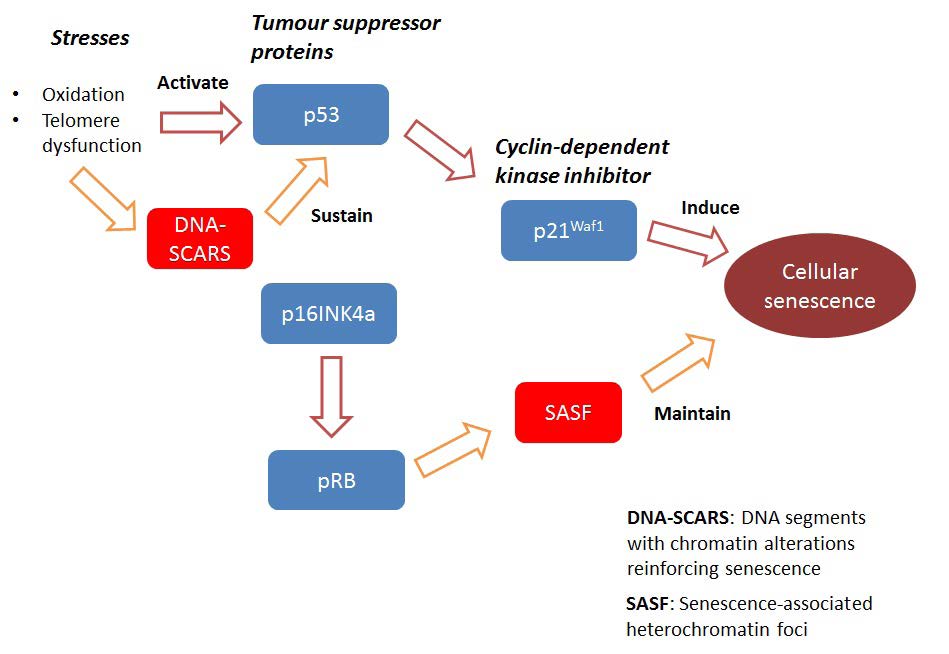
It was originally observed that cells that undergo senescence often do not divide even in the presence of mitogenic stimuli. Genomic stress from the environment can induce DNA damage pathways which inhibit cell cycle progression. In cancer cells, two important pathways are the p53 and p16INK4a/pRB pathways. [2] p53, p16INK4a, and pRB are important tumour suppressor proteins. While the mechanisms are still unclear and the contributions of p53 and p16INK4a may differ in different cancer cell types, it is suggested that p53 may be important for the establishment of senescence while p16INK4a maintains it (Figure 1). [3,4]
Activation of p53 by stresses such as oxidation and telomere dysfunction lead to upregulation of the cyclin-dependent kinase (CDK) inhibitor p21Waf1 which, in addition to apoptosis, causes cell cycle arrest and senescence. The activity of p53 is sustained by stress-induced DNA damage response signals which come from DNA damage foci, also known as DNA segments with chromatin alterations reinforcing senescence (DNA-SCARS). On the other hand, p16INK4a is essential for maintenance of senescence via the activation of the retinoblastoma (pRB) tumour suppressor protein. The pRB protein helps form senescence-associated heterochromatin foci (SAHF) which can silence tumour-promoting genes. [4]
Cancer cells can be cleared by apoptotic cell death, however, those which are resistant to initial apoptosis may be diverted to alternate pathways such as senescence, where they face a number of possible outcomes. [5] Firstly, cancer cells that undergo senescence are still capable of being eliminated by apoptosis at a later time. Secondly, senescent cancer cells may go into a terminal proliferative arrest state. It has been suggested that there is significant cross-talk between terminally arrested cancer cells and the immune system. Prolonged terminal arrest can trigger the clearance of cancer cells via phagocytosis and immunogenic cell death by autophagy. [6] Alternatively, immune mediators such as cytokines may be required for the maintenance of terminal arrest. [7] Dysregulation of this cross-talk can potentially result in bypass of cellular senescence and escape of cancer cells. The second outcome is interesting in a therapeutic sense as the involvement of immunogenic cell death is likely to bring about more sustained control than apoptosis. Apoptosis generally does not trigger an inflammatory response and the fact that cancer cells may harbor apoptotic defects suggests that this method of tumour suppression is not efficient. In fact, there is recent evidence that apoptosis may not even be the predominant mode of cell death in most cells, implying that other modes of cell death should be considered in cancer therapy. [8]
Senescence-associated secretory phenotype
While senescent cells exist in a state of growth arrest, they are still metabolically active and secrete a number of cytokines, chemokines, growth factors, and proteases which have important tumour-suppressive and tumour-promoting consequences. This unique phenotype is known as senescence-associated secretory phenotype (SASP) and can be found in senescent cells with DNA-SCARS. [9] As mentioned above, terminal arrest may be maintained by certain immune mediators. [4] These immune mediators may be secreted in an autocrine manner and help reinforce growth arrest. Examples of these tumour-suppressive mediators include plasminogen activator inhibitor-1 (PAI-1), and insulin-like growth factor binding protein-7 (IGFBP-7). On the other hand, tumour-promoting mediators can be secreted in a paracrine manner and induce aggressive phenotypes in neighbouring cells. These include factors such as matrix metalloproteinases (MMPs), amphiregulin, vascular endothelial growth factor (VEGF), as well as growth-related oncogene-alpha and beta (GRO-α & GRO-β). [4]
Certain pro-inflammatory cytokines such as interleukin-6 and interleukin-8 (IL-6 and IL-8) have paradoxical effects on tumour progression and their exact role may depend on the immune contexture. Chronic low-level inflammation can promote tumour progression whereas an acute high-grade inflammatory response can result in tumour regression. [10] It is worth postulating that the stimulation of immunogenic cell death via the second outcome may actually assist in augmenting pro-inflammatory cytokine levels in the acute phase, leading to elimination of cancer cells. On the other hand, apoptosis does not promote adequate IL-6 and IL-8 levels to result in clearance of these cells. Instead, epithelial-to-mesenchymal transition and the cell migration/invasion effects of these cytokines may become dominant, resulting in metastatic phenotypes. Besides secreting chemokines to attract immune cells, senescent cells also express ligands for cytotoxic immune cells such as natural killer (NK) cells, allowing for immune-mediated clearance of cancer cells. [11] It would seem counterintuitive that cellular senescence can have tumour-suppressive and tumour-promoting effects at the same time. How then, can we reconcile these paradoxical effects?
A temporal model of senescence
Cellular senescence is not a phenomenon restricted to cancer cells. In fact, it is a highly conserved process also found in normal cell types such as fibroblasts, and is involved in tissue repair as well as age-related degeneration. [12] Many of the tumour-promoting secreted factors found in the SASP are actually required for tissue regeneration. For example, VEGF is involved in angiogenesis while MMPs are required for degrading of fibrotic tissues found in damaged tissues. [13] Similarly, ageing tissues are characterised by low levels of chronic inflammation which can be mediated by factors such as IL-6 and IL-8. [14] The SASP is therefore a changing entity which differs in its secretory repertoire depending on the context it is expressed in. Rodier and Campisi proposed a model in which the senescent phenotype can be organised temporally. [15] In this model, the senescent phenotype increases in complexity with time. The initiating event is an oncogenic stress which either results in immediate repair and recovery of cells or induction of senescence. Once senescence occurs, cells are terminally arrested, resulting in tumour suppression. The SASP is then activated and IL-1α is secreted. This cytokine binds to the IL-1 receptor and induces a signalling cascade which leads to the activation of transcription factors such as NF-kB and C/EBPβ. This in turn simulates SASP factors such as IL-6, IL-8, and matrix metalloproteinases (MMPs) which are involved in both tissue repair and tumour progression. At the same time, pro-inflammatory cytokines such as IL-6 and IL-8 may increase to such high levels that they feed back and reinforce tumour suppression.
In addition, senescent cells may express a number of cell surface ligands and adhesion molecules which attract immune cells and result in clearance. During the later stages of senescence, the SASP phenotype is tuned down through the expression of microRNAs such as mir146a and mir146b so as to prevent the persistence of an acute inflammatory response. [16] However, the consequence is a chronic inflammatory state which can be perpetuated by imperfect immune clearance. A small number of senescent cells persist and contribute to chronic inflammation via their pro-inflammatory cytokines, which can eventually lead to the formation of an ageing phenotype. This phenotype is characterised by impaired functionality and increased vulnerability to cell death. It is apparent from this model that there is a delicate balance between different SASP phenotypes and imperfect immune processes can easily tilt the balance towards detrimental outcomes such as tumour progression and ageing phenotypes.
Susceptibility to tumour progression is not unexpected considering that important tumour suppressive proteins such as p53 and p16INK4a are often deficient or defective in cancer cells. [17] Although the defects in p53 and/or p16INK4a can be hurdles, these ‘weaknesses’ also provide unique opportunities for therapeutic interventions. In fact, cellular senescence might have originated foremost as a beneficial biological response. From an evolutionary perspective, it is suggested that senescence could have evolved to promote tumour suppression and tissue repair in young organisms. [4] These activities were selected as they are necessary for organismal survival in early harsh environments. However, unselected activities such as tumour progression and aging still occur as survival to old age is rare in harsh environments, and therefore selection against these detrimental activities is weak and tends to decline with age. It is therefore quite likely that senescence was meant to be a major tumour-suppressive mechanism and not simply a ‘backup’ plan to the more widely recognised apoptotic cell death.
Cellular senescence as a clinical strategy
While cellular senescence was initially thought to be irreversible in normal cells, a few studies have suggested that this process is reversible in cancer cells under the right conditions. For example, studies focusing on the tumour suppressors p53, pRB, and p16 found that suppression of these proteins in fibroblasts led to the reversal of the senescent phenotype. [18] Similarly, lung cancer cells were able to escape senescence through the up-regulation of Cdc2/Cdk1 and subsequently increased production of survivin, a protein involved in cell resistance to chemotherapy drugs such as paclitaxel. [19] This potentially implicates senescent cells as a repository for re-emergence of carcinogenesis. However, it should be noted that that there is a lack of evidence which suggests that cell-cycle re-entry is a sign of recovery of full proliferative capacity. Cells which re-enter the cell cycle may still be subjected to cell death by apoptosis or mitotic catastrophe at a later stage. [20]
In solid tumours, the use of chemotherapy alone yields a disease response rate of 20-40% and complete tumour eradication is often difficult to achieve. Considering that most anti-cancer agents kill by apoptotic cell death, this seems to suggest that apoptosis may be limited in its clinical efficacy. [21] Furthermore, regardless of whether cellular senescence is reversible or not, in vivo analysis of treatment responses in primary lymphomas have shown that senescence improves the outcome of cancer therapy despite the lack of intact apoptotic machinery. [17] One of many possible reasons for the improved outcome could be the prevention of cancer stem cells (i.e. precursor cancer cells) via the inhibition of mechanisms similar to induced pluripotent stem (iPS) cell (i.e. stem cells generated from adult tissue) reprogramming. [21] This is because potent inducers of senescence such as p53 and p16INK4a are also potent inhibitors of iPS reprogramming. There is also potential for senescence-based therapies to yield synergistic and additive treatment effects as conventional modalities such as chemotherapy and radiotherapy can induce ACS. [22] Therefore, attempts should be made to further consider senescence as a potential treatment strategy.
There are a number of possible directions that can be pursued in a senescence-based strategy. Firstly, the activity of tumour suppressor proteins and senescence-inducers such as p53 can be enhanced. This can be attained through p53 stabilisation or mutant p53 reactivation. p53 stabilisation was found to be mediated by small molecules known as nutlins. These molecules inhibit the E3 ubiquitin-protein ligase MDM2, which is a potent inhibitor of p53. Similarly, restoration of p53 was achieved by compounds such as the pro-apoptotic factor PRIMA-1MET and DNA intercalator ellipticine, which induce structural changes in the mutant protein and promote transcription of p53 targets . [23,24] Another possible target could be the inhibition of cell cycle progression via CDK inhibitors. One of the first CDK inhibitors to be tested in clinical trials is flavopiridol, which has been shown to exert tumour-suppressive effects in a number of malignancies such as colon and prostate cancer. [25] Flavopiridol, in certain doses, also appears to enhance treatment response when used in conjunction with standard chemotherapy agents, illustrating the proof of concept that senescence can augment existing treatment modalities. More recently, studies have investigated the use of statins in patients after neoadjuvant chemotherapy. Statins were shown to down-regulate key cell cycle mediators such as Cdk1, cyclin B1, and survivin, and up-regulate the CDK inhibitor p27. [21] However, antagonistic effects were also observed when statins were administered alongside chemotherapy due to escape from senescence. These observations suggest that the effects of statins need to be examined further, particularly in relation to their use before, during, or after chemotherapy. Besides modulation of p53 function and the use of CDK inhibitors, senescence can also be induced by inhibition of important oncogenes such as MYC. [26] MYC over-expression in tumours is associated with a poor prognosis when chemotherapy is used. By inhibiting MYC through small molecule inhibitors such as 10058-F5 and its derivatives, the expression of a number of genes involved in cell proliferation can be suppressed, contributing to cellular senescence. [27]
Conclusion
In summary, cellular senescence may be a viable strategy for oncological control. Although its therapeutic potential was first recognised through its ability to bring about permanent growth arrest of cancer cells, this viewpoint is too simplistic. Cellular senescence is in fact a dynamic process characterised by a SASP which evolves in complexity with time. The reversibility or irreversibility of cellular senescence depends on the immune context and delicate processes that regulate senescence (e.g. immune clearance and deficiency of tumour suppressive proteins). Although senescence is dependent on multiple factors, we should consider it as a major tumour-suppressive mechanism alongside apoptosis. During carcinogenesis, subversion of anti-tumour responses is commonplace and should not be perceived simply as weaknesses in a clinical strategy. This is illustrated by the observation that cancer cells that do not apoptose can still subsequently undergo apoptosis at a later stage or are subjected to more immunogenic forms of cell death like autophagy. Senescence can therefore function as a potent failsafe tumour-suppressive mechanism. On the contrary, therapeutic interventions should anticipate and augment existing barriers to tumour progression. In senescence, a number of possible solutions such as p53 enhancement, CDK inhibitors and oncogene inhibition provide reason for optimism and should be investigated further.
Acknowledgements
None.
Conflict of interest
None declared.
Correspondence
K Ho: koho2292@uni.sydney.edu.au
References
[1] Te Poele RH, Okorokov AL, Jardine L, Cummings L, Joel SP. DNA damage is able to induce senescence in tumour cells in vitro and in vivo. Cancer Res 2002; 62(6):1876-83.
[2] Miladi-Abdennadher I, Abdelmaksoud-Damak R, Ayadi L, Khabir A, Amouri A, Frikha F, et al. Expression of p16INK4a, alone or combined with p53, is predictive of better prognosis in colorectal adenocarcinoma in Tunisian patients. Appl Immunohistochem Mol Morphol 2011; 19(6):562-8.
[3] Yan Q, Wajapeyee N. Exploiting cellular senescence to treat cancer and circumvent drug resistance. Cancer Biol Ther 2010; 9(3):166-75.
[4] Campisi J. Cellular senescence: putting the paradoxes in perspective. Curr Opin Genet Dev 2011; 21(1):107-12.
[5] Chitikova ZV, Gordeev SA, Bykova TV, Zubova SG, Pospelov VA, Pospelova TV. Sustained activation of DNA damage response in irradiated apoptosis-resistant cells induces reversible senescence associated with mTOR downregulation and expression of stem cell markers. Cell Cycle 2014; 13(9):1424-39.
[6] Petrovski G, Ayna G, Majai G, Hodrea J, Benko S, Madi A, et al. Phagocytosis of cells dying through autophagy induces inflammasome activation and IL-1β release in human macrophages. Autophagy 2011; 7(3):321-30.
[7] Acquavella N, Clever D, Yu Z, Roelke-Parker M, Palmer DC, Xi L, et al. Type 1 cytokines synergize with oncogene inhibition to induce tumour growth arrest. Cancer Immunol Res 2015; 3(1):37-47.
[8] Tait SW, Ichim G, Green DR. Die another way—non apoptotic mechanisms of cell death. J Cell Sci 2014; 127(10):2135-44.
[9] Rodier F, Munoz DP, Teachenor R, Chu V, Le O, Bhaumik D, et al. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci 2011; 124(1):68-81.
[10] Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140(6):883-99
[11] Iannello A, Raulet DH. Immunosurveillance of senescent cancer cells by natural killer cells. Oncoimmunology 2014; 3:e27616. doi:10.4161/onci.27616.
[12] Kortlever RM, Bernards R. Senescence, wound healing and cancer: the PAI-1 connection. Cell Cycle 2006; 5(23):2697-703
[13] Kornek M, Raskopf E, Tolba R, Becker U, Klockner M, Sauerbruch T, et al. Accelerated orthotopic hepatocellular carcinomas growth is linked to increased expression of pro-angiogenic and prometastatic factors in murine liver fibrosis. Liver Int 2008; 28(4):509-18.
[14] Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J. Cell surface-bound IL-1α is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci USA 2009; 106(40):17031-36.
[15] Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol 2011; 192(4):547-56.
[16] Bhaumik D, Scott GK, Schokrpur S, Patil CK, Orjalo AV, Rodier F, et al. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging (Albany NY) 2009; 1(4): 402-11.
[17] Schmitt CA, Fridman JS, Yang M, Lee S, Baranov E, Hoffman RM, et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 2002; 109(3):335-46.
[18] Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumour suppression. Annu Rev Pathol 2010; 5:99-108.
[19] Wang Q, Wu PC, Roberson RS, Luk BV, Ivanova I, Chu E, et al. Survivin and escaping in therapy-induced cellular senescence. Int J Cancer 2011; 128(7):1546-58.
[20] Khalem P, Dorken B, Schmitt CA. Cellular senescence in cancer treatment: friend or foe. J Clin Invest 2004; 113(2):169-74.
[21] Wu PC, Wang Q, Grobman L, Chu E, Wu DY. Accelerated cellular senescence in solid tumor therapy. Exp Oncol 2012; 34(3):298-305
[22] Gewirtz DA, Holt SE, Elmore LW. Accelerated senescence: an emerging role in tumour cell response to chemotherapy and radiation. Biochem Pharmacol 2008; 76(8):947-57.
[23] Zandi R, Selivanova G, Christensen CL, Gerds TA, Willumsen BM, Poulsen HS. PRIMA-1Met/APR-246 induces apoptosis and tumour growth delay in small cell lung cancer expressing p53. Clin Cancer Res 2011; 17(9):2830-41.
[24] Deane FM, O’Sullivan EC, Maguire AR, Gilbert J, Sakoff JA, McCluskey A, et al. Synthesis and evaluation of novel ellipticines as potential anti-cancer agents. Org Biomol Chem 2013; 11(8):1334-44.
[25] Motwani M, Li X, Schwartz GK. Flavopiridol, a cyclin-dependent kinase inhibitor, prevents spindle inhibitor-induced endoreduplication in human cancer cells. Clin Cancer Res 2000; 6(3):924-32.
[26] Reimann M, Lee S, Loddenkemper C, Dorr JR, Tabor V, Aichele P, et al. Tumour stroma-derived TGF-beta limits myc-driven lymphomagenesis via Suv39h1-dependent senescence. Cancer Cell 2010; 17(3):262-72.
[27] Wanner J, Romashko D, Werner D, May EW, Peng Y, Schulz R, et al. Reversible linkage of two distinct small molecule inhibitors of myc generates a dimeric inhibitor with improved potency that is active in myc over-expressing cancer cell lines. Plos One 2015; 10(4):e0121793.