4th Year MBBS, University of Notre Dame
Management of chronic post-surgical pain: an overview
Alexandra Richards
Thursday, August 31st, 2017
4th Year MBBS, University of Notre Dame
Chronic pain is an anticipated complication of any surgery despite comprehensive treatment modalities to combat it. The development of chronic pain is attributable to a larger variety of inherent risks. Due to both the individual and social costs of chronic, unremitting pain, the value in preventing its development is paramount. Considering the complex pathophysiology of pain, chronic post-surgical pain (CPSP) development requires a multimodal understanding that involves understanding the physiological, psychological, and social circumstances of the patient. Prevention and management of CPSP starts preoperatively, addressing the patient’s risk factors and expectations to anticipate and create a more personalised plan for pain control. Intraoperative measures include local anaesthesia and pharmacological analgesic therapies. postoperatively, a multidisciplinary approach utilising both pharmacological and non-pharmacological strategies can be used. Pharmacological treatments include individualised opioid-based patient controlled analgesia in conjunction with prostaglandin inhibitors, central nervous system pain receptor modulators, and nerve blocks. Non-pharmacological management includes transcutaneous electrical stimulation and acupuncture. A good understanding of how CPSP develops can aid in managing CPSP that can result in better control of chronic pain.
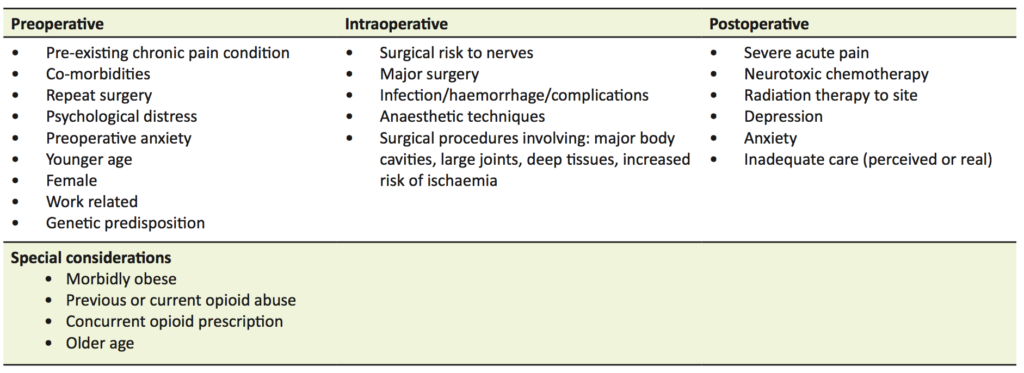
Pain in the perioperative period is a common and anticipated complication of surgery. Pain can be attributed in part to surgical factors such as nerve injury, inflammation, and infection. In addition, chronic pain development is dependent on a larger variety of putative risks that include past medical history of chronic pain and perioperative anxiety (Table 1) [1]. Chronic post-surgical pain (CPSP) is classified by the International Classification of Disease 11, as persisting pain for at least three months after surgery or tissue trauma, with the exclusion of other or pre-existing causes (such as infection and malignancy) [1,2]. The presentation of CPSP is often variable and may occur in relation to deep tissue or skin trauma at the surgical site, referred from viscero-somatic convergence, or related to nerve injury during surgery [3,4].
While epidemiological accounts vary, the populations at risk of developing CPSP are variable depending on the types of surgery and the likelihood of nerve injury. Current literature indicates that CPSP occur in 10-50% of surgical cases. In orthopaedic surgery related CPSP, up to 5% of all surgical patients report severe disabling pain at one year postoperatively, and a further 10% report lesser pain [1,3,4]. In a UK study of 5000 patients considering frequency and cause of chronic pain in the secondary care setting, 22% of outpatients attributed surgery as the major cause of their chronic pain [3,5]. As chronic pain is difficult and costly to manage and has a major impact on quality of life and productivity, the socioeconomic health burden is potentially enormous considering the volume of surgeries performed annually [1,5].
Formerly, conceptualisation of chronic pain was limited to uni-factorial models of biomedical causality. These models sought to explain pain as corresponding directly to bodily damage with severity being a measurement of extent (for example, using a visual analogue scale or numeric pain rating scale), rather than patient interpretation of injury [1,6]. However, the lack of a clear relationship between extent of tissue/nerve damage and pain severity indicates there is a psychological and sociological component to pain, with the additional influences of motivation and secondary gains (family, work, and “the sick role”) [6]. These aspects were heavily enforced by operant learning factors, which develop into “pain behaviours” of avoidance, and cognitive factors based around beliefs and expectations of pain post-surgery [7]. Patients who have a past medical history including emotional or psychiatric stressors, have increased work-related injuries and claims, negative attitudes to treatments, and previous chronic pain diagnosis are at an increased risk of developing chronic pain syndromes [1,8]. To demonstrate this point, a large 2007 prospective surgical cohort study using preoperative psychological questionaries (Item-36 Short Form Health Survey) with postoperative acute pain scores for 625 patients undergoing minor, intermediate, and major surgeries, found that fear of the long-term consequences of surgery predicted increased pain in the six month postoperative follow-up period, independent of the type of procedure and other somatic factors [7].
Generally, surgical pain can be attributed to three main mechanisms: inflammation, direct nerve injury, and increased sensitisation [1,2]. Inflammatory pain arising from tissue trauma and ischaemia is an unavoidable aspect of most surgeries [4]. Although the release of local inflammatory mediators like tumour necrosis factor alpha and interleukins 1 and 6 are needed to a certain degree for healing, they can result in hyperalgaesia (augmented sensitivity) and allodynia (misperception of non-painful stimuli) long after the expected healing time from surgery [1,4]. These outcomes are attributed to peripheral nerve sensitisation and are often managed with anti-inflammatory medications both intraoperatively and on an outpatient basis [4]. In conjunction, pain developing from direct nerve injury (compression, stretch, or transection) can have a similar presentation in addition to hyperpathia (exaggerated pain), paraesthesia/dysaesthesia (abnormal sensation) and even hypoesthesia (decreased sensation) [1,4]. For example, nerve injury arising from fetal head descent through the birth canal during labour may result in compression or stretch of lumbosacral nerve roots resulting in radiculopathy from 8% elongation, while 15% elongation can result in axon disruption and axonotmesis [7].
Pain sensitisation is also a major precipitating factor for the transition from acute to chronic pain. The pathophysiology of sensitisation is attributed to increased excitability of both central and peripheral nerve fibres (in addition to decreased inhibition from dorsal horn spinal neurons) [1,10]. Centrally, sensitisation is linked to upregulation of N-methyl-D-aspartate (NMDA) receptors in the dorsal horn causing the “wind-up” phenomenon of pain, with peripheral changes from prolonged inflammation or opioid exposure linked to ‘hyperalgesic priming’ at the afferent sensory nerve level [1,2]. Ectopic activity in transected nerves has also been associated as the underlying cause for the spontaneous pain characteristics of some neuropathic states that involves maladaptive plasticity within the nerve nociceptive system post-injury [1]. It is predominantly owing to this sensitisation (with input from patient psychology) that the major risk factor for developing CPSP is severe acute post-surgical pain, making acute pain management of foremost importance for CPSP prevention [1,2].
Owing to its complexity, pain management warrants a comprehensive and surgery specific multimodal approach [5]. This starts preoperatively with patient risk factors and expectations addressed to anticipate potential complications and acceptable therapies. Furthermore, following procedure specific guidelines produces better clinical outcomes with appropriate discharge and rehabilitation planning incorporating a pain clinic follow-up [2]. For example, open colorectal procedures may benefit from thoracic epidurals to reduce postoperative pain, nausea, and vomiting, while laparoscopic abdominal procedures, with minimal tissue injury, often do not require the same cover [11]. These considerations should be made preoperatively with patient expectations taken into account and the risk of neuropathic/nerve injury considered for procedure appropriate analgesia [2].
Intraoperatively, local and systemic therapies can be used to target the aforementioned biomedical risks and can be further broken into nociceptive and neuropathic targets. Systemic therapies often involve opioid and limited non-opioid options for nociceptive pain, with opioid use being limited by adverse effects (such as respiratory depression and vomiting) [2].
Patient-controlled analgesia (PCA) remains a cornerstone of postoperative pain management, with early postoperative intravenous opioids providing better analgesia than conventional parenteral opioid regimens, with greater patient satisfaction particularly for nociceptive pain [2,11]. There is little evidence that any particular opioid delivered via PCA is superior to another in regard to analgesic or adverse effects in general, but individual patients may tolerate one opioid better than another, and safety of administration can be impacted by hospital staff education [12].
In regards to non-opioid analgesics, there is fair evidence to support their complementary use with opioid analgesics [2]. Medications in this category that target nociceptive pathways include non-steroidal anti-inflammatory drugs (NSAIDs) which are superior to paracetamol (although combining both increases efficacy), and selective COX-2 inhibitors (a subtype of NSAIDs) which offer further advantages over their non-selective counterparts in particular with regard to platelet dysfunction, blood loss, and renal impairment [2,11]. Other multimodal analgesic options that can assist in neuropathic pain management involve intravenous local anaesthetics (such as lignocaine), which has been shown to reduce opioid requirements after abdominal surgery and to decrease the risk of nausea, vomiting, and duration of postoperative ileus, and so decrease the length of hospital stay [12,13]. Locally, lignocaine can also be injected proximally to surgical sites intraoperatively as a preventative somatic analgesia, although a 2005 meta-analysis of 66 randomised controlled trials (comparing preoperative analgesic interventions with similar postoperative analgesic interventions via the same route) and a 2005 randomised controlled trial assessing pain relief in laparoscopic gynaecological surgery suggested the use of pre-emptive local infiltration was associated with a more limited, but still beneficial effect on post-surgical visceral pain [14,15].
The importance of multimodal approaches targeting not only the nociceptive, but also the neuropathic and central neurons can be seen in the prevention of wind-up phenomena and central sensitisation [6,12]. For this, therapies targeting not only opioid, but also substance P, calcitonin gene-related peptide, aspartate, glutamate, gamma-aminobutyric acid (GABA), and NMDA receptors can be used to target pain on multiple levels of the pain pathway [1,2]. Examples of neuropathic treatments include perioperative use of gabapentin and pregabalin, which have both been shown to decrease postoperative opioid requirements [13,16]. Similarly, a meta-analysis of 29 randomised controlled trials indicated a small yet significant decrease in CPSP with intra- and postoperative ketamine use [8]. Furthermore, peri-operative intravenous ketamine also reduces opioid use and postoperative nausea and vomiting compared with placebo, in addition to being cost effective and useful in opioid-tolerant patients [2,17].
Concurrently, the use of regional blocks or neuraxial methods such as femoral nerve blocks may reduce the use and side effects of systemic opioids whilst facilitating early mobilisation and recovery (thereby reducing the psychological impact of illness) [8]. Risk of infection exists, but is predominantly preventable by sterile precautions. Likewise, the risk of neuropathy from this procedure is low and countered generally by the use of ultrasound guidance [11]. Additionally, while regional blocks present an increased risk of procedural complications depending on the block used (for example, brachial plexus blocks risk pneumothorax), these are far rarer than complications of opioid use and often outweigh them on a clinical outcome basis for controlling neuropathic pain [11,18].
In terms of patient education, better pain relief is achieved by structured preoperative education and written information, rather than routine information with generalised verbal discussion [2]. Although identification of preoperative risk factors may assist in targeted patient education and expectation management (Table 1), other interventions, such as pre-surgical hypnosis and music therapy, have been found to be reliable for decreasing CPSP as an outcome indicated by six month follow-up surveys and postoperative pain surveys [1, 7]. Identification of factors that will make pain management more difficult, such as obesity, history of opioid abuse, and current opioid use may assist in appropriate pre and post-surgical management [1,2].
Finally, non-pharmacological methods including transcutaneous electrical stimulation and acupuncture have been shown to reduce postoperative pain, particularly in the setting of back surgery and ambulatory knee surgery when compared to placebo, with the aforementioned psychological methods (distraction, music, and video) being of potential use in paediatric populations. However, the evidence for these is limited and variable in the literature [2,11].
In conclusion, CPSP is a common, inherently complex, and costly complication of surgery. Managing chronic post surgical pain from a multimodal multidisciplinary approach may improve pain control.
References
- Mariano E, Fanciullo G, Crowley M. Management of acute perioperative pain. UpToDate Clinical Topic Reviews. 2016 Jun 13.
- Schug SA, Palmer GM, Scott DA, Halliwell R, Trinca J. Acute pain management: scientific evidence, 2015. Med J Australia. 2016;204(8):315-7.
- Bruce J, Quinlan J. Chronic post surgical pain. Rev Pain. 2011;5(3):23.
- Therapeutic Guidelines Ltd. The transition from acute to chronic pain: risk factors for postsurgical pain syndromes. eTG Complete: Analgesic. 2012, Mar.
- Macrae WA. Chronic post-surgical pain: 10 years on. Brit J Anaesth. 2008;101(1):77-86.
- Turk DC, Okifuji A. Interdisciplinary approach to pain management: philosophy, operations, and efficacy. In: Ashburn MA, Rice LJ, editors. The management of pain. Baltimore: Churchill-Livingstone; 1998.
- Peters ML, Sommer M, Rijke JM, Kessels F, Heineman E, Patijn J, et al. Somatic and psychologic predictors of long-term unfavorable outcome after surgical intervention. Ann Surg. 2007;245(3):487-94.
- Theunissen M, Peters ML, Bruce J, Gramke HF, Marcus MA. Preoperative anxiety and catastrophizing: a systematic review and meta-analysis of the association with chronic postsurgical pain. Clin J Pain. 2012;28(9):819-41.
- Flores AJ, Lavemia CJ, Owens PW. Anatomy and physiology of peripheral nerve injury and repair. Am J Orthop. 2000;29(3):167-78.
- Johansen A, Romundstad L, Nielsen CS, Schirmer H, Stubhaug A. Persistent postsurgical pain in a general population: prevalence and predictors in the Tromsø study. Pain. 2012;153(7), 1390-6.
- Corke P. Postoperative pain management. Aust Prescr. 2013;36(6).
- Gilron I. Antidepressant drugs for postsurgical pain: current status and future directions. Drugs. 2016;76(2):159-67.
- Dualé C, Ouchchane L, Schoeffler P, Group EI, Dubray C. Neuropathic aspects of persistent postsurgical pain: a French multicenter survey with a 6-month prospective follow-up. J Pain. 2014;15(1):24-e1.
- Ong CK, Lirk P, Seymour RA, Jenkins BJ. The efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysis. Anesth Analg. 2005;100(3):757-73
- Ghezzi F, Cromi A, Bergamini V, Raffaelli R, Crotti S, Segredini R, et al. Preemptive port site local anesthesia in gynecologic laparoscopy: a randomized, controlled trial. J Minim Invas Gyn. 2005;12(3):210-5.
- Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg. 2012;115(2):428-42.
- Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. The Cochrane Library. 2013 Jan 1.
- Haroutiunian S, Nikolajsen L, Finnerup NB, Jensen TS. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain. 2013;154(1):95-102.

