Perioperative glycaemic control in diabetic surgical patients – review
Andrew Zhang
Tuesday, December 1st, 2015
Glycaemic control around the time of surgery is a critical part of surgical care in diabetic patients. There is a high prevalence of diabetes mellitus worldwide, and the disease is becoming more common in both medical and surgical patients. Even in patients without diabetes, surgery disrupts usual diabetic management and glucose homeostasis, often resulting in perioperative hyperglycaemia. Hyperglycaemia has been associated with increased postoperative mortality and morbidity, as well as worse surgical outcomes in both cardiac and non-cardiac surgery. Published evidence suggests that outcomes can be improved in perioperative patients by closer management and control of glucose.
Despite early studies in the intensive care unit (ICU) setting, subsequent trials were not able to demonstrate improved outcomes with the use of intensive insulin therapy, which aimed for stricter glycaemic control (4.5–6.0 mmol/L) that was closer to physiological ranges. Whilst the optimal blood glucose concentrations are still unknown, current literature supports the use of moderately strict glycaemic control (5.0–10.0 mmol/L) via a basal-bolus insulin regimen, so as to balance the risks of inducing hypoglycaemia with the benefits of avoiding hyperglycaemia.
Diabetes mellitus is a highly prevalent group of metabolic diseases worldwide, and a significant proportion of surgical patients are diabetic. [1] An important component of diabetic perioperative management is glucose control, as glucose homeostasis is easily disrupted during periods of physical stress and illness. Recent studies have shown that hyperglycaemia during surgery is not a benign condition like it was once considered to be, and that treatment results in reduced mortality and morbidity. This literature review will focus on the current understandings of the effects of diabetes, how hyperglycaemia can affect clinical outcomes in the surgical setting, and the present consensus on the management of blood glucose in diabetic patients perioperatively.
Methods
This literature review is constructed as an unsystematic narrative review. The search for current literature was performed through the Ovid Medline database, the PubMed database, and the Cochrane Library. The following search terms and their related terms were used: perioperative glycaemic control, perioperative hyperglycaemic, perioperative diabetic management, intensive insulin therapy, sliding-scale insulin, basal bolus. The articles evaluated were limited to publication between January 1st, 2000 and March 1st, 2015. Articles published were restricted to the English language and to the adult surgical population. Given the breadth of literature on the topic, only major influential studies were selected for review. Studies performed on highly specific populations were excluded. Relevant retrospective observational studies, RCTs, and meta-analyses were included for analysis. Published review articles and editorials were examined for major influential studies. Any relevant in-text citations were also considered for inclusion. In total, forty-seven (n = 47) articles were selected for full text retrieval after abstract screening.
Background
Epidemiology
According to the Australian Institute of Health and Welfare, approximately 900 000 Australians have diabetes. [2] However, it has been estimated that up to half of all cases remain undiagnosed. [3] Similarly, the International Diabetes Federation estimates that diabetes prevalence in the adult Australian community is 9.99%. [4] Type 2 diabetes is the most common variant, accounting for 85-90% of all diabetics. [5] An audit across eleven hospitals in metropolitan Melbourne indicated that 24.7% of all inpatients had diabetes, with prevalence ranging from 15.7% to 35.1% in different hospitals. [6] Given the predicted exponential rise in obesity over the next decade and the current trend of an ageing population, projections suggest that 3.3 million Australians will have type 2 diabetes by 2031. [7]
Pathophysiology of diabetes
Although pathogenesis differs for the various forms of diabetes mellitus, hyperglycaemia is an underlying mechanism by which the disease can cause long-term complications. Diabetes is characterised by a lack of, or reduced effectiveness of, endogenous insulin, which then results in elevated fasting blood glucose concentrations and an inadequate response to glucose loads. Glucose homeostasis is tightly regulated, with normal blood glucose values being maintained within a narrow range between 4.4–7.8 mmol/L. [8] Chronic concentrations above 7.0 mmol/L are capable of producing end organ damage. [9]
Left untreated, diabetes mellitus is a disease associated with acute and chronic organ dysfunction and failure. Persistent hyperglycaemia leads to morbidity mainly through damaging medium and large-sized arteries (macrovascular disease) and causing capillary dysfunction in end organs (microvascular disease). Macrovascular disease increases the risk of developing ischaemic heart disease, cerebrovascular disease, and peripheral vascular disease, while microvascular disease results in diabetic retinopathy, nephropathy, and neuropathy. [10]
Diabetes and surgery
Given the high prevalence of diabetes seen in the community and hospitals, we can expect a significant proportion of those who present for surgery to have the diagnosis. Diabetic complications such as ischaemic heart disease and diabetic eye disease also increase the likelihood of requiring surgical interventions, and it has been estimated that 50% of all diabetic patients will undergo surgery at some stage. [11] The prevalence of cardiovascular diseases, including hypertension, coronary artery disease and stroke, are two to four times higher in diabetic patients, compared to non-diabetics. [12] Diabetes is also the leading cause of end-stage renal failure, adult-onset blindness, and non-traumatic lower extremity amputations.
In addition, diabetes puts patients at a higher perioperative risk for adverse outcomes when compared to non-diabetics. Mortality has been reported to be up to 50% higher than that of the non-diabetic population. [13] Diabetic patients are also more likely to develop postoperative infections, arrhythmias, acute renal failure, ileus, stroke, and myocardial ischaemia. [14-16] Due to the wide range of complications that can occur, diabetic patients have a 45% longer length of stay postoperatively, with higher health care resource utilisation, compared with non-diabetic patients. [17]
Diabetic patients are prone to dysregulation of glucose homeostasis, especially during surgical stress or critical illness. Since most surgical patients will need to fast prior to surgery, there is often considerable disruption to their usual diabetes management routine. About 20% of elective surgical patients demonstrate impaired fasting blood glucose concentrations. [1] Other factors such as postoperative infections and emesis can all lead to labile blood glucose concentrations. Meanwhile, both surgery and anaesthesia produce a hypermetabolic stress response by elevating the levels of stress hormones and inflammatory cytokines such as catecholamines, cortisol, growth hormone, and TNF-α. [18] These hormones increase blood glucose concentrations by upregulating hepatic gluconeogenesis and glycogenolysis, as well as exacerbate insulin resistance and decrease insulin secretion. [18]
Discussion
Effects of perioperative hyperglycaemia and benefits of glycaemic control
Hyperglycaemia is a prevalent phenomenon in surgical patients. One study found that 40% of non-cardiac surgery patients had a blood glucose concentration >7.8 mmol/L, with 25% of those patients having a blood glucose concentration >10.0 mmol/L. [19] Perioperative hyperglycaemia was once considered to be a beneficial physiological adaptive response to surgery and critical illness, intended to supply energy to vital organs. This is now largely known to be untrue, with observational studies and randomised controlled trials indicating that improvement in glycaemic control results in lower morbidity and mortality, shorter length of stay, and fewer complications such as nosocomial infections, postoperatively. Outside of surgery, hyperglycaemia has also been associated with worse outcomes in critically ill, hospitalised patients. [20] Patients who are hyperglycaemic following a stroke demonstrate worse functional recovery and higher mortality compared to patients with normal glycaemic control. [21]
Retrospective observational studies
An observational study on patients undergoing non-cardiac surgery by Frisch et al. demonstrated that perioperative hyperglycaemia is associated with significantly increased risk of pneumonia, sepsis, urinary tract infection, skin infection, acute renal failure and death during the postoperative period. [19] Ramos et al. found a correlation between blood glucose concentrations and the rate of postoperative infection and length of hospital stay in general and vascular surgical patients. The study observed that every 2.2 mmol/L rise in postoperative blood glucose concentration above 6.1 mmol/L resulted in an increase in the infection rate by 30%. [22] In cardiac surgery, Gandhi et al. observed that intraoperative hyperglycaemia is an independent risk factor for post-operative complications, including death. [23] Schmeltz et al. demonstrated that the use of a combination of IV and subcutaneous insulin to improve glucose control in cardiac surgery reduced the mortality and infection rates among diabetic patients to those of non-diabetic patients. [24]
Hyperglycaemia has been shown to be the significant risk factor for perioperative morbidity and mortality, rather than diabetes itself. A retrospective cohort study based on 11,633 patients by Kwon et al. found that perioperative hyperglycaemia was associated with a near doubling in the risk of infection, mortality, and operative complications in both diabetic and non-diabetic general surgical patients. [25] A retrospective study by Doenst et al. concluded that a high peak blood glucose concentration during cardiopulmonary bypass was an independent risk factor for death and morbidity in diabetic patients. [26]
Prospective randomised controlled trials
A prospective randomised controlled study of surgical ICU patients by Van den Berghe et al. in 2001 (first Leuven study) demonstrated significantly reduced morbidity and mortality in critically ill patients when the blood glucose concentrations were maintained between 4.4–6.1 mmol/L via an intravenous insulin infusion. [27] In another randomised prospective study by Lazar et al., 141 diabetic cardiac surgery patients were assigned to either moderately tight glycaemic control (6.9–11.1 mmol/L) with a glucose-insulin-potassium (GIK) regimen, or to standard therapy (<13.9 mmol/L) using intermittent subcutaneous insulin. [28] The GIK patients had a lower incidence of atrial fibrillation and a shorter postoperative length of stay, compared to patients receiving standard therapy. The intervention was commenced prior to anaesthesia, and only continued for 12 hours postoperatively. Interestingly, the GIK patients were able to demonstrate a survival advantage two years postoperatively, with decreased episodes of recurrent myocardial ischaemia and fewer recurrent wound infections. This suggests that moderately tight control even for a brief period can make substantial differences to long-term outcomes.
The Diabetes Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study by Malmberg et al., which looked at 620 diabetic patients post-acute myocardial infarction, reported a 29% reduction in the 1-year mortality rate in patients who were randomised to receive intensive glucose management (mean blood glucose concentration of 9.6 mmol/L at 24 hours) when compared to patients assigned to receive conventional treatment (mean blood glucose concentration of 11.7 mmol/L at 24 hours). [29]
The question of whether the insulin therapy itself or the treatment of hyperglycaemia resulted in benefit has not been fully answered, as the metabolic and cellular actions of insulin may contribute to the beneficial outcomes. Insulin therapy has been shown to improve dyslipidaemias and prevent endothelial dysfunction and hypercoagulability in critically ill patients. [30] Treating a patient with insulin causes arterial vasodilation and capillary recruitment, via activation of the nitric oxide pathway and improves myocardial perfusion. [31] However, the first Leuven study found that the positive effects of intensive insulin therapy were related to the lower blood glucose concentrations, rather than insulin doses. [27]
Intensive versus conventional glycaemic control
Beyond avoidance of marked hyperglycaemia and hypoglycaemia, the optimal perioperative glucose targets are unclear. Conventional glycaemic control targets blood glucose concentrations <10.0 mmol/L, and there has been considerable controversy over the safety and efficacy of intensive insulin therapy (IIT), which aimed at a much lower and narrower concentration between 4.5–6.0 mmol/L. Despite early results, which suggested decreased mortality and other advantages of intensive glucose control, [27] later investigations found no benefits or increased mortality when hyperglycaemia was aggressively treated with insulin. [32-33] The current consensus is that intensive control does not actually confer any benefits with regards to mortality, but increases the risk for hypoglycaemia, which is a potentially life-threatening complication. [34] The brain is an obligate glucose metaboliser, hence neurons are particularly vulnerable to low blood glucose concentrations. Even brief periods of hypoglycaemia (i.e. blood glucose concentration <2.2 mmol/L) can induce arrhythmias, cardiac events, and brain injury. [35]
The first Leuven study published in 2001 by Van den Berghe et al. demonstrated significant reductions in morbidity and mortality (by 34%) in over 1500 surgical ICU patients with tight glycaemic control (4.4–6.1 mmol/L) when compared to conventional control (<10–11.1 mmol/L). [27] Intensive insulin therapy (IIT) was also shown to decrease the duration of mechanical ventilation and ICU length of stay. However, there were many study limitations that could have affected the validity of the results. Many subsequent randomised controlled trials and meta-analyses that were published contrasted with the initial Leuven study, finding no benefit when IIT was used for glycaemic control, as well as a significantly higher risk of hypoglycaemia. [32-34]
A second Leuven study published in 2006 by Van den Berghe et al. was a randomised controlled trial comparing IIT and conventional therapy in 1200 medical ICU patients, and it did not demonstrate any mortality benefit with intensive insulin therapy, while observing more prevalent hypoglycaemic events in the treatment group. [32] Kujik et al. observed that intensive glucose control in the perioperative period has no clear benefit on short-term mortality in patients undergoing major vascular surgery, and recommended that moderate tight glucose control be regarded as the safest and most efficient approach to patients undergoing surgery. [36] Duncan et al. found that in cardiac surgery, although severe intraoperative hyperglycaemia (>11.1 mmol/L) was associated with higher risk of mortality and morbidity, blood glucose concentrations closest to normoglycaemia (average of 7.78 mmol/L or less) were also associated with increased mortality and morbidity. [37] In fact, the lowest risk of adverse outcomes was observed in the range between 7.8–9.4 mmol/L, suggesting that mild hyperglycaemia was better tolerated than strict control. The association of tight blood glucose control with worse outcomes was observable despite rare episodes of hypoglycaemia, which suggests that there are factors other than hypoglycaemia that could contribute to the poor outcomes of intensive glucose control.
The largest and most definitive study to date is the Normoglycaemia in Intensive Care Evaluation – Survival Using Glucose Algorithm Regulation (NICE-SUGAR) study, which was a multicentre, international, randomised controlled trial aimed at comparing intensive insulin therapy (4.5–6 mmol/L) with conventional treatment (8–10 mmol/L). [33] The study reported a higher 28-day and 90-day mortality rate in surgical ICU patients who received IIT, with significantly more severe hypoglycaemia in those patients. The authors were not able to demonstrate a difference in hospital or ICU length of stay, length of mechanical ventilation, or the need for renal replacement. In contrast to the initial Leuven study, mortality rates were higher in the IIT group (27.5% vs 24.9%). The NICE-SUGAR trial also reaffirmed a higher incidence of hypoglycaemia in the IIT group.
A Cochrane meta-analysis of 12 randomised trials (1403 patients with diabetes) comparing intensive (blood glucose concentration of <6.7 or <8.3 mmol/L) versus conventional (variable) glycaemic control by Buchleitner et al. also found that intensive glycaemic control has no significant effect on infectious complications, cardiovascular events, or mortality, except for increasing the number of hypoglycaemic episodes. [34] Given the current data available from randomised controlled clinical trials, the authors concluded that intensive glycaemic control protocols with near-normal blood glucose targets cannot be generally recommended for patients with diabetes undergoing surgery.
Basal-bolus versus sliding scale insulin
Insulin is generally the preferred method of treatment for inpatients as it is an effective medication for immediate control of hyperglycaemia in the hospital setting. The dose can be titrated more rapidly than that of oral hypoglycaemic agents, and it does not have a dose ceiling. Insulin can be delivered either subcutaneously or intravenously as a continuous infusion, and the use of sliding-scale insulin (SSI) has traditionally been the mainstay of hyperglycaemia therapy. However, recent studies have shown that the use of SSI alone is insufficient in providing adequate glycaemic control, and that a combination of basal and supplemental insulin is a more effective approach.
The combined use of basal insulin (i.e., intermediate- to long-acting insulin) together with short- or rapid-acting insulin before meals is able to better mimic physiological patterns of glucose control. The RABBIT 2–Medical trial by Umpierrez et al. demonstrated an improvement in glycaemic control with basal-bolus insulin in 130 diabetic insulin-naïve medical patients, with no increase in the number of hypoglycaemic events. [38] The subsequent RABBIT 2–Surgical trial, which is a multi-institutional randomised trial that looked at 211 type 2 diabetic general surgical patients, also found improved glycaemic control and reduced hospital complications with the basal-bolus regimen when compared to the sliding-scale insulin regimen. [39] The most recent evidence suggests that both medical and surgical type 2 diabetic patients with poor glycaemic control (blood glucose concentration >10 mmol/L or HbA1c >7.5%) should be treated with the basal-bolus insulin regimen. [40]
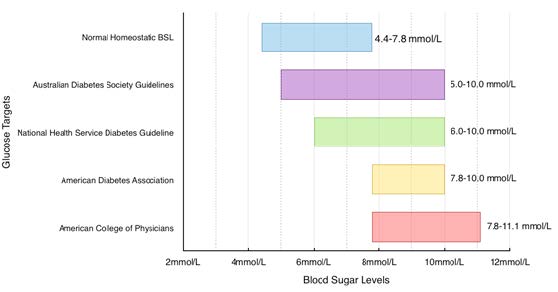
Figure 1. Recommended perioperative BSL targets.
Given that studies have failed to show a benefit and some even show increased mortality with intensive insulin therapy, the management of glucose concentrations has undergone drastic changes in the past decade. Current recommendations from the UK, US, and Australia all recommend a similar range of blood glucose concentrations (Figure 1). Most guidelines tolerate mild hyperglycaemia as it reduces the potential for developing hypoglycaemia. Insulin therapy in both general medical and surgical settings should consist of a mixture of basal, prandial, and supplemental insulin (basal-bolus), instead of the sliding-scale regimen. However, differences in these recommendations indicate that the most optimal blood glucose concentrations are still unknown.
Preoperative insulin therapy should focus on obtaining good glycaemic control, while avoiding episodes of hypoglycaemia. The Endocrine Society’s Clinical Guidelines recommend a fasting blood glucose concentration of <7.8 mmol/l and a random blood glucose concentration of <10.0 mmol/l for the majority of hospitalised patients with non-critical illness. [41] For avoidance of hypoglycaemia, therapy should be reassessed when blood glucose concentration falls below 5.6 mmol/L. Similarly, the National Health Service Diabetes Guideline published in 2012 by Dhatariya et al. recommends blood glucose concentrations between 6.0–10.0 mmol/L, while accepting 4.0–12.0 mmol/L. [42] The Australian Diabetes Society currently recommends a blood glucose target of 5.0–10.0 mmol/L in both the ICU and non-ICU settings. [43] The American Diabetes Association and the American Association of Clinical Endocrinologists currently recommend commencing insulin therapy for critically ill patients with persistent hyperglycaemia (>10.0 mmol/L), and to aim for a blood glucose target range between 7.8–10.0 mmol/L, [44] while the American College of Physicians recommends 7.8–11.1 mmol/L for critically ill patients. [45]
The mainstay of type 2 diabetes therapy is oral hypoglycaemic agents, such as metformin and sulfonylureas. Most guidelines suggest withholding oral anti-diabetic agents and non-insulin injectable medications on the day of surgery but not before. For major surgeries, metformin should be withheld for at least 24 hours. This is because oral diabetic medications can potentially produce hypoglycaemia during the fasting period prior to surgery, as well as systemic effects that may affect postoperative outcomes. For example, sulfonylureas can interfere with the opening of cardiac KATP channels, which increases the risk for myocardial ischaemic injury. [46] Metformin can potentially induce lactic acidosis if renal function is impaired. [47] However, ceasing anti-diabetic therapy too early may compromise glucose control, hence short- or medium-duration insulin should be used to treat acute hyperglycaemia during the operative period. Oral hypoglycaemic agents should not be restarted until adequate and regular oral intake is resumed.
The majority of patients receiving insulin therapy should use a basal-bolus insulin schedule. The long-acting agents are aimed at providing a steady, basal level of insulin while the shorter-acting bolus insulin is used to counter acute increases in blood glucose. It is important to note that not only type 1 diabetics, but all insulin dependent patients, will require insulin perioperatively, despite their fasting status. This is because these patients are insulin deficient and require consistent basal insulin replacement to prevent unchecked gluconeogenesis and ketosis.
Conclusion
Hyperglycaemia has been shown to produce deleterious effects in multiple body systems, both acutely and chronically. Studies indicate that adequate glycaemic control during the perioperative period is beneficial for both short-term and long-term surgical outcomes. While the optimal target blood glucose range is still unclear, the literature supports the use of moderately strict glycaemic control for the management of hyperglycaemia in surgical patients. The use of basal-bolus insulin is preferred over the more traditional sliding-scale insulin for its efficacy and safety. With the current trend of rising diabetes incidence in Australia, maintaining good glycaemic control during the perioperative period will become an increasingly important challenge faced by health professionals.
Acknowledgements
Professor Kate Leslie, Head of Research, Department of Anaesthesia and Pain Management, Royal Melbourne Hospital, Melbourne, Australia for her critical review and helpful comments and suggestions.
Conflict of interest
None declared.
Correspondence
A Zhang: zazhang@student.unimelb.edu.au
References
[1] Hatzakorzian R, Bui H, Carvalho G, Shan WL, Sidhu S, Schricker T. Fasting blood glucose levels in patients presenting for elective surgery. Nutrition. 2011;27(3):298-301.
[2] Australian Institute of Health and Welfare (AIHW). Diabetes prevalence in Australia: detailed estimates for 2007-2008. Diabetes series no. 17. Cat. no. CVD 56. Canberra: AIHW; 2011. [Cited 12 Aug 2014] Available from: http://www.aihw.gov.au/publication-detail/?id=10737419311
[3] Valentine NA, Alhawassi TM, Roberts GW, Vora PP, Stranks SN, Doogue MP. Detecting undiagnosed diabetes using glycated haemoglobin: an automated screening test in hospitalised patients. Med J Aust. 2011;194(4):160-4.
[4] International Diabetes Federation. IDF Diabetes Atlas. 6th ed. Brussels, Belgium: IDF; 2013. [Cited Feb 2015]. Available from: http://www.idf.org/diabetesatlas
[5] World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications; Part 1: Diagnosis and classification of diabetes mellitus. Department of Noncommunicable Disease Surveillance, Geneva, 1999. (WHO/NCD/NCS/99.2).
[6] Bach LA, Ekinci EI, Engler D, Gilfillan C, Hamblin PS, MacIsaac RJ, et al. The high burden of inpatient diabetes mellitus: the Melbourne Public Hospitals Diabetes Inpatient Audit. Med J Aust. 2014;201(6):334-8.
[7] Vos T, Goss J, Begg S, Mann N. Australian Burden of Disease and Injury Study, Projected Health Care Costs Report. University of Queensland and AIHW, Canberra, 2007.
[8] Engelgau MM, Narayan KM, Herman WH. Screening for type 2 diabetes. Diabetes Care. 2000;23(10):1563-80.
[9] Bash LD, Selvin E, Steffes M, Coresh J, Astor BC. Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absence of albuminuria and retinopathy: Atherosclerosis Risk in Communities (ARIC) Study. Arc Intern Med. 2008;168(22):2440-7.
[10] Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26(2):77-82.
[11] Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, Schafer RG, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-91.
[12] Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the multiple risk factor intervention trial. Diabetes Care. 1993;16:434-44.
[13] Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53(8):2079-86.
[14] Godoy DA, Di Napoli M, Biestro A, Lenhardt R. Perioperative glucose control in neurosurgical patients. Anesthesiol Res Pract 2012; 2012: 690362
[15] Milaskiewicz RM, Hall GM. Diabetes and anaesthesia: the past decade. Br J Anaesth. 1992;68(2):198-206.
[16] Thourani VH, Weintraub WS, Stein B, Gebhart SS, Craver JM, Jones EL, et al. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. Ann Thorac Surg. 1999;67:1045-52.
[17] Hall GM, Page SR. Diabetes and surgery. In: Page SR, Hall GM, editors. Emergency and hospital management. London: BMJ Publishing; 1999.
[18] Meneghini LF. Perioperative management of diabetes: Translating evidence into practice. Cleve Clin J Med. 2009;76(Suppl 4):S53-9.
[19] Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, Gatcliffe C, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010;33(8):1783-8.
[20] Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79:992-1000.
[21] Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32(10):2426-32.
[22] Ramos M, Khalpey Z, Lipsitz S, Steinberg J, Panizales MT, Zinner M, et al. Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg. 2008;248(4):585-91.
[23] Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, Williams BA, et al. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin Proc. 2005;80(7):862-6.
[24] Schmeltz LR, DeSantis AJ, Thiyagarajan V, Schmidt K, O’Shea-Mahler E, Johnson D, et al. Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy. Diabetes Care. 2007;30:823-828.
[25] Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8-14.
[26] Doenst T, Wijeysundera D, Karkouti K, Zechner C, Maganti M, Rao V, et al. Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2005;130(4):1144.
[27] Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359-67.
[28] Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation. 2004;109(12):1497-502.
[29] Malmberg K. Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group. BMJ. 1997;314(7093):1512-5.
[30] Van den Berghe G. How does blood glucose control with insulin save lives in intensive care? J Clin Invest. 2004;114(9):1187-95.
[31] Duncan AE. Hyperglycemia and perioperative glucose management. Curr Pharm Des. 2012;18(38):6195-203.
[32] Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449-61.
[33] Investigators N-SS, Finfer S, Chittock DR, Su SY, Blair D, Foster D, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283-97.
[34] Buchleitner AM, Martinez-Alonso M, Hernandez M, Sola I, Mauricio D. Perioperative glycaemic control for diabetic patients undergoing surgery. Cochrane Database Syst Rev. 2012;9:CD007315.
[35] Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010;33(6):1389-94.
[36] van Kuijk JP, Schouten O, Flu WJ, den Uil CA, Bax JJ, Poldermans D. Perioperative blood glucose monitoring and control in major vascular surgery patients. Eur J Vasc Endovasc Surg. 2009;38(5):627-34.
[37] Duncan AE, Abd-Elsayed A, Maheshwari A, Xu M, Soltesz E, Koch CG. Role of intraoperative and postoperative blood glucose concentrations in predicting outcomes after cardiac surgery. Anesthesiology. 2010;112(4):860-71.
[38] Umpierrez GE, Smiley D, Zisman A, Prieto LM, Palacio A, Ceron M, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181-6.
[39] Umpierrez GE, Smiley D, Jacobs S, Peng L, Temponi A, Mulligan P, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-61.
[40] Umpierrez GE, Smiley D, Hermayer K, Khan A, Olson DE, Newton C, et al. Randomized study comparing a Basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care. 2013;36(8):2169-74.
[41] Umpierrez GE, Hellman R, Korytkowski MT, Kosiborod M, Maynard GA, Montori VM, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16-38.
[42] Dhatariya K, Levy N, Kilvert A, Watson B, Cousins D, Flanagan D, et al. NHS Diabetes guideline for the perioperative management of the adult patient with diabetes. Diabet Med. 2012;29(4):420-33.
[43] Australian Diabetes Society. Peri-operative Diabetes Management Guidelines. 2012. [Cited 10 September 2014]. Available from: https://diabetessociety.com.au/documents/PerioperativeDiabetesManagementGuidelinesFINALCleanJuly2012.pdf
[44] Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American Association of Clinical Endocrinologists and American Diabetes Association Consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119-31.
[45] Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;154(4):260-7.
[46] Garratt KN, Brady PA, Hassinger NL, Grill DE, Terzic A, Holmes DR, Jr. Sulfonylurea drugs increase early mortality in patients with diabetes mellitus after direct angioplasty for acute myocardial infarction. J Am Coll Cardiol. 1999;33(1):119-24.
[47] Mercker SK, Maier C, Neumann G, Wulf H. Lactic acidosis as a serious perioperative complication of antidiabetic biguanide medication with metformin. Anesthesiology. 1997;87(4):1003-5.