Medication-induced acute angle-closure glaucoma: a case study
Allister Howie
Wednesday, April 1st, 2015
Acute angle-closure glaucoma, is an uncommon condition. It is an emergency associated with the potential for significant vision loss and unilateral blindness if not diagnosed and treated promptly. This case describes a classic presentation of angle-closure glaucoma, highlighting the potential of certain medications to precipitate acute angle-closure glaucoma in at-risk individuals. Although the incidence is uncertain, it is thought that a significant number of cases may be medication-induced, and so it is important to be aware of what medications may precipitate acute angle-closure and have a plan for assessing and managing this small but real risk. In addition, patients should be warned of possible ocular symptoms and advised to seek urgent medical attention if they occur. In a presentation of acute angle-closure glaucoma, the key management is urgent reduction of intraocular pressure and ophthalmology referral
Case
A 65-year-old female presented to her general practitioner with a painful, red left eye associated with blurred vision and nausea. She had commenced paroxetine for management of depression three weeks prior. On examination, best-corrected visual acuity was 6/19 in the left eye, 6/6 in the right. The left pupil was mid-dilated and fixed. Examination was otherwise normal. An urgent ophthalmology review was organised and a diagnosis of acute angle-closure glaucoma was made.
Though uncommon in Australia, acute angle-closure glaucoma (AACG) is a medical emergency that requires rapid diagnosis and reduction of intraocular pressure to prevent permanent vision loss. [1,2]
Discussion
Epidemiology
Glaucoma is the second leading cause of vision loss worldwide, with an estimated 79.6 million people to be affected in 2020. Though approximately 74% of cases worldwide are open-angle glaucoma, it is projected that 5.3 million people will be legally blind due to AACC in 2020, comparable to the 5.9 million estimated to be blind due to open-angle glaucoma. [2]
Pathogenesis
Overwhelmingly, the most common cause of angle closure crisis is pupillary block. Aqueous humour normally flows between the pupil and lens, from the posterior chamber to the angle of the anterior chamber of the eye, where it then drains across the trabecular meshwork. When the pathway between the lens and iris is blocked, aqueous accumulates behind the iris, pushing it anteriorly and blocking the trabecular meshwork, thus preventing aqueous drainage. [3] When this occurs, intraocular pressure (IOP) rapidly becomes elevated, frequently reaching pressures greater than 60 mmHg, rapidly causing glaucomatous optic neuropathy if untreated. [4] Eyes with pre-existing anatomic narrow angles are predisposed to acute angle-closure.
Medication-induced angle-closure
Medication-induced angle-closure has been reported to cause a significant proportion of AACC cases in developed countries. [5] Consequently, it is important to be aware of the risk when prescribing implicated medications. The underlying mechanism may be due to pupil dilatation (mydriasis) as a medication side effect, or due to choroidal effusion, causing swelling of the ciliary body and forward movement of the lens and iris towards the chamber angle. [4,6]
Ophthalmic mydriatics are a well-known precipitant of angle-closure crisis, but other medications with mydriatic effects also carry risk. Importantly, this includes several classes of antidepressants: selective-serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants and serotonin–noradrenaline reuptake inhibitors. [7] Visual disturbance has frequently been a cause of SSRI withdrawal and it has been suggested that SSRI-induced intraocular pressure elevation may be underestimated. [8]
Any medication with sympathetic or anticholinergic effects has theoretical potential to precipitate angle-closure in at-risk eyes. Other drugs implicated include phenothiazine antipsychotics, antihistamines, benzhexol, over-the-counter medications containing phenylephrine, nebulised ipratropium bromide and salbutamol. [4,9]
Topiramate, an anticonvulsant commonly used to treat epilepsy and for migraine prophylaxis, has commonly been reported as a precipitant of AACC in the literature due to choroidal effusion. A report suggested topiramate may be the most common cause of AACC in individuals under the age of 40. [11] The risk is thought to be due to the sulfur component of the drug. Other sulfur-containing medications, including acetazolamide, have been reported as precipitants, though only rarely, so should therefore not be used to treat topiramate-induced angle-closure. [6,9,10]
Drug-induced acute angle-closure usually develops soon after initiation of treatment, and generally within 30 days. Whilst AACC classically presents with a unilateral red eye with pain and reduced vision, bilateral presentations may occur and are more common in medication-induced cases. If symptoms are consistent with angle-closure crisis, a high index of suspicion must be maintained. [11,12]
Clinical implications
Clinicians should be aware of medications associated with increased risk of angle-closure glaucoma, and consider and warn the patient of this small possibility when initiating these medications. [13] The risk of causing angle-closure when dilating pupils is very low (estimated 1 in 20 000) and mydriatics should always be used when performing a complete fundus examination. A good choice of medication to minimise risk is tropicamide 0.5%. [14]
It would be impractical for an ophthalmologist to review every patient before prescribing many of the associated medications, however, a brief history of ocular symptoms should be taken and the risk profile of the patient stratified. [9]
Acute angle-closure risk factors include:
- Advanced age
- Asian ethnicity
- Severe hyperopia (beware of the patient wearing thick glasses)
- Known shallow anterior chamber, or occludable angle
- Family history of blindness suspicious for angle-closure glaucoma
The oblique flashlight test, a simple way to estimate anterior chamber depth, should be performed (Figure 1). A light is shined onto the temporal iris. If the anterior chamber is deep, the iris will be uniformly illuminated. Shadowing of the nasal iris indicates the anterior chamber may be shallow, increasing risk of anterior chamber angle occlusion. The test is only 45.5% specific, but is 82.7–91.7% sensitive and may be performed rapidly. [15,16]
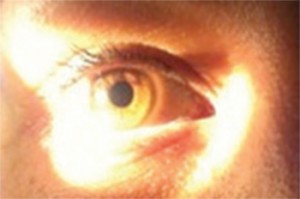
deep anterior chamber
Patients should be warned of ocular symptoms and their significance at first prescription of implicated medications, particularly if identified to be at high risk. If at high risk, an ophthalmology referral should be considered to evaluate the degree of openness of the angle that is prone to angle-closure. [9,13] On review after initiating implicated medications, it is important to ask patients whether they have experienced any ocular symptoms. [17]
It is important to note that open-angle glaucoma is a separate condition and patients with this condition should not be denied medications associated with angle-closure glaucoma. [18]
Treating angle-closure glaucoma
Immediate treatment goals are rapid reduction of intraocular pressure and symptomatic relief. Intravenous mannitol 5–10 mL/kg of 20% solution, given over 30 minutes, will cause a rapid, temporary reduction in intraocular pressure. [19] Intravenous or oral acetazolamide may also be required. Topical medications, including timolol, prednisolone, and brimonidine, may be used. [10] A miotic, such as pilocarpine, is used to constrict the pupil. This will pull the peripheral iris away from the angle, thereby opening drainage through the trabecular meshwork. Antiemetics and analgesics should be given as needed, and the patient should be supine, in an attempt to prevent further anterior movement of the lens. Any medications possibly contributing should be ceased. [5,12]
Definitive treatment is laser iridotomy, performed by an ophthalmologist. An opening is made in the peripheral iris, allowing free flow of aqueous between posterior and anterior chambers, allowing equilibration of pressure between the chambers, thus preventing the occurrence of pupillary block. Both eyes are treated, even in unilateral presentations, as the fellow eye is likely to have the same narrowed angles, which increases the risk of angle-closure. [3,11]
Consent
Informed consent was obtained from the patient for publication of this case report. Informed consent was obtained from individuals photographed for the purposes of this report.
Conflict of Interest
None declared.
References
[1] Wensor M, McCarty C, Stanislavsky Y, Livingston P, Taylor H. The prevalence of glaucoma in the Melbourne Visual Impairment Project. Ophthalmology. 1998;105(4):733-9.
[2] Quigley H, Broman A. The number of people with glaucoma worldwide in 2010 and 2020. Brit J Ophthalmol. 2006;90(3):262-7.
[3] Masselos K, Bank A, Francis IC, Stapleton F. Corneal indentation in the early management of acute angle closure. Ophthalmology. 2009;116(1):25-9.
[4] Subak-Sharpe I, Low S, Nolan W, Foster PJ. Pharmacological and environmental factors in primary angle-closure glaucoma. Brit Med Bull. 2010;93:125-43.
[5] Lachkar Y, Bouassida W. Drug-induced acute angle closure glaucoma. Curr Opin Ophthalmol. 2007;18(2):129-33.
[6] Chen T, Chao C, Sorkin J. Topiramate induced myopic shift and angle closure glaucoma. Brit J Ophthalmol. 2003;87(5):648-9.
[7] de Guzman M, Thiagalingam S, Ong P, Goldberg I. Bilateral acute angle closure caused by supraciliary effusions associated with venlafaxine intake. Med J Australia. 2005;182(3):121-3.
[8] Costagliola C, Parmeggiani F, Sebastiani A. SSRIs and intraocular pressure modifications: evidence, therapeutic implications and possible mechanisms. CNS drugs. 2004;18(8):475-84.
[9] Razeghinejad M, Pro M, Katz L. Non-steroidal drug-induced glaucoma. Eye (Lond). 2011;25(8):971-80.
[10] Ybarra M, Rosenbaum T. Typical migraine or ophthalmologic emergency? Am J Emerg Med. 2012;30(5):831.
[11] Pokhrel P, Loftus S. Ocular emergencies. Am Fam Physician. 2007;76(6):829-36.
[12] Amerasinghe N, Aung T. Angle-closure: risk factors, diagnosis and treatment. Prog Brain Res. 2008;173:31-45.
[13] Cackett P. Funduscopy: to dilate or not? Other drugs can cause partial pupil dilatation. Brit Med J. 2006;332(7534):179.
[14] Liew G, Mitchell P, Wang JJ, Wong TY. Fundoscopy: to dilate or not to dilate? Brit Med J. 2006;332(7532):3.
[15] Yu Q, Xu J, Zhu S, Liu Q. A role of oblique flashlight test in screening for primary angle closure glaucoma. Yan Ke Xue Bao. 1995;11(4):177-9.
[16] He M, Huang W, Friedman D, Wu C, Zheng Y, Foster P. Slit lamp-simulated oblique flashlight test in the detection of narrow angles in Chinese eyes: the Liwan eye study. Invest Ophth Vis Sci. 2007;48(12):5459-63.
[17] Lai J, Gangwani R. Medication-induced acute angle closure attack. Hong Kong Med J. 2012;18(2):139-45.
[18] Razeghinejad M, Myers J, Katz L. Iatrogenic glaucoma secondary to medications. Am J Med. 2011;124(1):20-5.
[19] Mannitol. Australian Medicines Handbook [internet]. 2013 [cited 2014 March 1] Available from: http://www.amh.net.au/online/
